| 014P London, UK Pharmacology 2016 |
Microtubules regulate β adrenoceptor-mediated relaxations in rat arterial smooth muscle
Introduction: Microtubules regulate G protein-coupled receptor signalling in various cell types. In vascular smooth muscle, activation of β-adrenoceptors produces cAMP to mediate a vasorelaxation. Given the importance of this pathway in vascular smooth muscle cells, we investigated the effect of microtubule-disrupting agents on β-adrenoceptor signalling in rat renal and mesenteric arteries.
Methods: We performed isometric tension and sharp microelectrode recordings on segments of 12- to 15-week-old male Wistar rat renal and mesenteric arteries (a total of 27 rats were used). Individual artery segments were pre-contracted with 3 µM methoxamine, a α1-adrenergic receptor agonist and, increasing concentrations of isoprenaline was applied. This was performed before and after 90 mins incubation with microtubule disrupting agents.Experiments were performed in accordance with the European Union legislation for the protection of animals used for scientific purposes, and approved by the National Animal Experiments Inspectorate. Reagents colchicine ((S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl)acetamide), nocodazole ([5-(2-Thienylcarbonyl)-1H-benzimidazol-2-yl]carbonic acid, methyl ester), XE991 (10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone), pinacidil and R-L3 (5-(2-Fluorophenyl)-1,3-dihydro-3-(1H-indol-3-ylmethyl)-1-methyl-2H-1,4-benzodiazepin-2-one) were purchased from Torics (Abingdon, U.K). S-1 ((S)-N-[1-(3-morpholin-4-yl-phenyl)-ethyl]-3-phenyl-acrilamide) was synthesised by Neurosearch (Ballerup, Denmark). All other drugs and salts were purchased from Sigma.Results: Application of the microtubule disruptors colchicine (300 and 500 µM) or nocodazole (10 µM) for 90 mins enhanced the relaxations of renal and mesenteric arteries to the β-adrenoceptor agonist isoprenaline (0,1-3,0 µM) compared to incubation with DMSO (figure 1; n>5 for all groups). Subsequently, we found the colchicine-enhanced relaxations to isoprenaline in the mesenteric arteries were associated with a more hyperpolarised membrane potential compared to DMSO treated arteries (-61.6 ± 5.2 mV vs -35.4 ± 2.5 mV, respectively; n=5; P=0.0019). The increased membrane hyperpolarisation in the presence of colchicine implies a greater efflux of K+ from the cell. We therefor tested whether blockade of Kv7 channels with XE991 (10,10-bis(4-Pyridinylmethyl)-9(10H)-anthracenone dihydrochloride) would attenuate the enhanced isoprenaline-mediated relaxation following incubation with colchicine. In both arteries, XE991 inhibited the colchicine- and nocodazole-enhanced isoprenaline responses (n>5 for all groups). In addition, colchicine improved the relaxations to the Kv7.2-Kv7.5 activator, S-1 ((S)-N-[1-(3-morpholin-4-yl-phenyl)-ethyl]-3-phenyl-acrylamide), in both renal (n=5) and mesenteric artery (n=5) segments compared to DMSO incubation; whereas the Kv7.1 channel activator, R-L3 (5-(2-Fluorophenyl)-1,3-dihydro-3-(1H-indol-3-ylmethyl)-1-methyl-2H-1,4-benzodiazepin-2-one) (n=5), and KATP channel activator, pinacidil (n=5), were unaffected by microtubule disruption.
Conclusions: This study is the first to show that microtubules can restrict the β-adrenoceptor-mediated relaxations in arterial smooth muscle. Following microtubule disruption, the β-adrenoceptor-mediated relaxations improved and, we identified this enhancement to be predominantly due to increased recruitment of the Kv7 voltage-gated potassium channels.
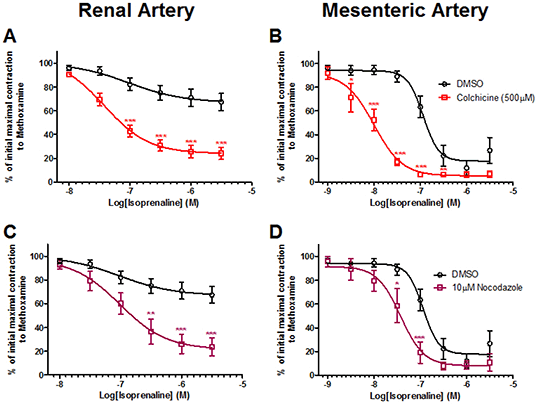
Figure 1:The effect of colchicine (500 μM) and nocodazole (10 μM) compared to DMSO on isoprenaline relaxations in rat renal and mesenteric arteries (n>5 for all points). *, **, *** denote signifcance of P<0.05, P<0.01, P<0.001, respectively.

