| 016P London, UK Pharmacology 2016 |
Effects of chronic administration of the GABAB receptor agonist baclofen on body weight and food intake in the mouse
Introduction: We have previously demonstrated that intraperitoneal (ip) administration of the GABAB receptor agonist baclofen reduces body weight in rats without significantly affecting daily food intake1. The present study, which was ethically approved, was undertaken to extend these observations to another animal species, namely the mouse.
Methods: Male C57B/10 mice (n=28, body weight: 20 - 28g) were housed in pairs in cages with free access to food and water. The animals were injected daily for 15 days with either saline or baclofen (4 or 8 mg kg-1; i.p). Body weight and food intake were measured 24h after each injection. The results were analysed by repeated measures ANOVA and the post hoc Tukey test.
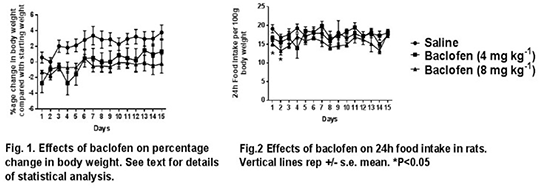
Results: There was a significant effect of treatment on body weight gain (F(2,21)=4.88, P<0.02; Fig.1). Post-hoc tests revealed that 4 mg kg-1 dose of baclofen significantly reduced body weight gain during the first 5 days, while the 8 mg kg-1 dose reduced body weight gain during the 15 days of the study. The lower dose of baclofen had no effects on 24h food intake throughout the study. The 8 mg kg-1 dose produced small but significant decreases in 24h food intake on days 1 and 2 (P<0.05) that were probably due to the initial depressant effects of the drug on behaviour1,2 but had no significant effects thereafter (see Fig.2)
Conclusions. The results of this study extends previous findings in rat to another rodent species and show that systemic administration of the GABAB receptor agonist baclofen reduces body weight gain in the mouse without affecting daily food intake. These results give further credence to the hypothesis that GABAB receptor agonists decreases body weight by increasing metabolic rate1,2.
References:
1. Patel SM et al (2010) Eur J Pharmacol 635: 129 - 134.
2. Ebenezer IS et al. (2016) Proceedings of the British Pharmacological Society at http:/www.pA2online.org/abstracts/Vol13Issue3abstract177P.pdf