| 034P London, UK Pharmacology 2016 |
Molecular modelling and molecular dynamics simulations of a new berberine derivative as a dual RAF inhibitor for treatment of drug resistance acquired in melanoma
Introduction: Melanoma is a type of skin cancer designed by abnormal formation of melanocytes. BRAF oncoprotein is associated with almost 66% of melanomas. BRAF is a major target for targeted therapy of cancer and is the most frequently mutated protein kinase in human cancers. The most common BRAF mutation is called V600E (1). BRAFV600E inhibitors such as vemurafenib have shown drug resistance (2). In the current study, compounds that were structurally similar to berberine (a plant-based alkaloid) have been studied to predict the most effective compounds on BRAF and CRAF.
Method: Using molecular modelling, thermodynamics parameters were computed for virtually screened compounds following the Rule of five. To validate the results, molecular dynamics (MD) simulations were performed with Gromacs 5.0.1, and MD parameters such as radial distribution function (RDF), root-mean-square deviation (RMSD), and energies (potential, enthalpy, etc.) were computed and compared among different ligands. The interactions have been modelled and binding efficiency indices were then calculated. In all in silico experiments, three independent protein crystals were used, and vemurafenib and ATP were used as positive control.
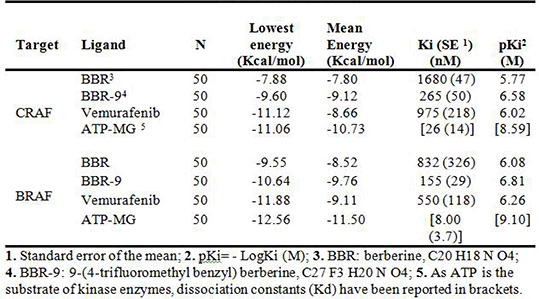
Results: In this study three compounds were predicted to have the highest affinity to BRAF and CRAF among the studied berberine (BBR) derivatives (Table 1). The interactions and the involved residues were depicted for both enzymes. As both enzymes have a homology in their kinase domain, Lys483 and Lys375 were predicted to be one of the most important residues involved in BRAF (Figure 1) and CRAF dockings, respectively. π-π and cation-π interactions were determined as the most important interactions involved in berberine docking as well as hydrogen bonding.
Conclusion: In the present study, we sought to find drug candidates, which simultaneously inhibit BRAF and CRAF kinases. In contrast to BBR, we found that BBR-9 predicted to play as stronger dual RAF inhibitor. Furthermore, RAF kinases are located at the central point of the MAP kinase pathway and downstream of receptor tyrosine kinases and RAS which are mutated in more than 50% of carcinomas. Therefore, BBR-9 indirectly inhibits mutated RTKs and RAS by interrupting RAF/RAS pathway.
References:
1. Puzanov I, Amaravadi RK, McArthur GA, et al. (2015) Eur J Cancer 51:1435–1443.
2. Zubrilov I, Sagi-Assif O, Izraely S, et al. (2015) Cancer Lett 361:86–96.
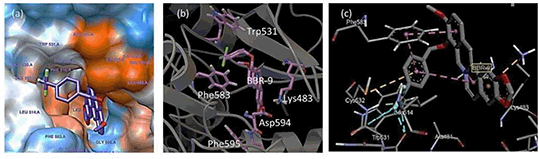
Figure 1. Interactions and neighboring residues in BBR-9/BRAFV600E docking. Purple-colored lines are showing T-shaped π-π interaction. BBR-9 shows inter- and intra- molecular π-π interactions.
Table 1. Comparative docking information for the best berberine derivative docked to BRAF and CRAF.