| 035P London, UK Pharmacology 2016 |
Morphine tolerance in dorsal root ganglion nociceptors is induced by mediators in colon tissue of chronic morphine treated mice
Introduction: Chronic morphine exposure in mice compromises epithelial tight junction integrity, allowing bacterial translocation to the gut wall.1 Bacterial products and secondary immunoinflammatory responses may then modulate the activity of dorsal root ganglion (DRG) nociceptors with terminal processes in the gut wall. The clinical utility of opioids is greatly limited by the development of tolerance to their analgesic effects, and DRG nociceptors play a critical role in opioid antinociception. However, it is unknown whether the altered gut microenvironment following chronic morphine exposure can directly influence tolerance in DRG nociceptors.
Method: Adult male Swiss Webster mice were implanted with a 75 mg morphine (MP) or placebo pellet (PP) for 5 days. Full circumference colon segments 5 mm in length were resected and incubated (37 °C) in 400 µL of neurobasal A medium containing 1% FBS, 1x B-27 supplement, 2 mM L-glutamine, 10 ng/mL GDNF, and penicillin/streptomycin/amphotericin B for 24 hours.2 The culture supernatants (400 µL) were then transferred to freshly isolated naïve DRG neuron cultures. An additional 200 µL of fresh medium was added to the cultures, which were incubated (37 °C) for 24 hours before performing patch-clamp investigations.
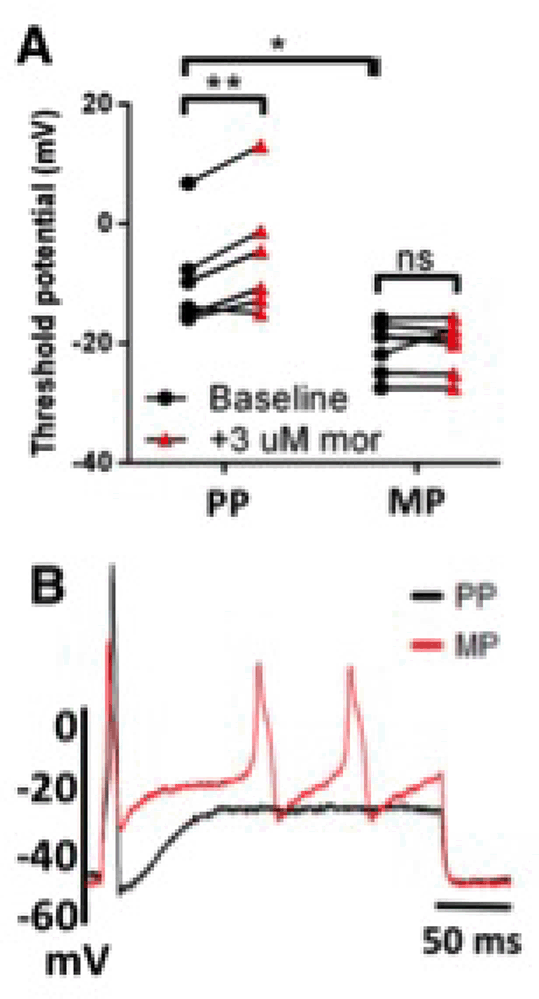
Results: DRG nociceptors exposed to supernatants from placebo-pelleted (PP) mice responded to 3 μM morphine superfusion with a positive shift in the action potential threshold (mean±SEM -9.32±3.48 to -5.23±4.23 mV), indicating reduced excitability (A). In contrast, nociceptors exposed to colon supernatants from morphine-pelleted (MP) mice did not respond to morphine challenge (-20.73±1.61 to -20.46±1.62 mV), indicating tolerance development. Hyperexcitability was further noted in DRG nociceptors exposed to supernatants from MP, but not PP, mice as double rheobase current injection produced multiple action potentials (B). | A: PP (N=5,n=6), MP (N=4,n=7). ns not significant, *p<0.05, **p<0.01 by two-way repeated-measures ANOVA with Bonferroni post-hoc analysis.
Conclusion: These findings suggest that chronic morphine treatment influences the chemical milieu of the colon wall in a way that promotes morphine tolerance development in DRG neurons. Future studies aim to identify the constituents of the supernatants (e.g., cytokines or bacterial products) causing the observed effects. Translational investigations using human colon biopsy samples would also give great credence to the relevance of these findings.
References:
1. Meng, J, et al. (2013). PLoS ONE. 8(1), e54040.
2. Valdez-Morales, EE, et al. (2013). Am J Gastroenterol. 108, 1634-1643.