Print version
Search Pub Med
| 039P London, UK Pharmacology 2016 |
A phase i study to determine the pharmacokinetic profile, safety and tolerability of sildenafil (REVATIO®) in cardiac surgery: the REVAKI-1 study.
Introduction: Acute kidney injury (AKI) is a common and severe complication of cardiac surgery. It increases mortality fourfold and there is no effective prevention or treatment. There is no effective prevention or Treatment-2.
Sildenafil citrate (Revatio®, Pfizer Inc), a phosphodiesterase type 5 inhibitor, inhibitor that has clinical efficacy in diseases characterised by endothelial dysfunction prevents post cardiac surgery AKI in pre-clinical studies-1.
However its use is contraindicated in patients with severe symptomatic cardiovascular disease and intraoperative sildenafil administration has not been reported previously in cardiac surgery.
Method: To assess the safety, pharmacokinetics and pharmacodynamics of intravenous sildenafil in cardiac surgery patients we conducted an open label, dose escalation study with 6 patients per dose level. The six doses were 2.5 mg, 5 mg or 10 mg as a bolus, either alone or followed by a 2 hour infusion of additionally 2.5 mg sildenafil. Key pharmakodynamic markers were obtained at baseline and during the treatment phase.
Results: Thirty six patients entered the trial, of which 33 completed it. The mean age was 69.9 years. One patient died during the surgery, two others were removed from the trial before dosing (all at dose level 5mg + 2.5 mg).
The pharmacokinetic profile of sildenafil cardiac surgery patients undergoing cardiopulmonary bypass (CPB) was similar to those reported in other patient groups[6,7]. For a dose of 10mg administered as a bolus followed by 2.5 mg administered over 2 hours the results were AUC∞ 537 ng h/mL, Cmax 189.4 ng/ml, and T1/2 10.5 hours. Plasma levels that are known to have clinical efficacy in other conditions were well tolerated during and after cardiac surgery with no serious adverse events related to the drug. Higher sildenafil doses stabilised post-surgery Nitric Oxide bioavailability.
Figure 1: Mean plasma concentrations of Sildenafil in cardiac surgery
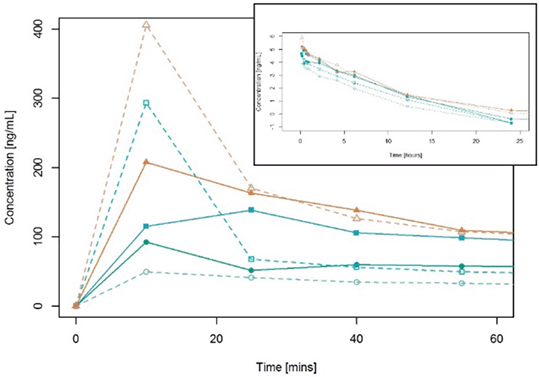
Figure 2: Time course of selected biomarkers following sildenafil treatment
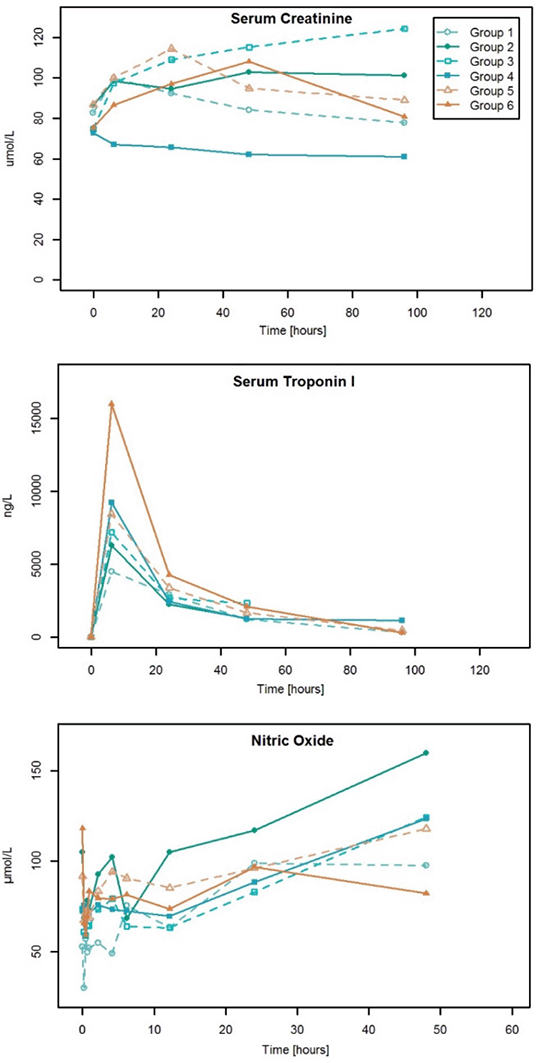
Conclusions: Pharmacokinetics of sildenafil during CPB were comparable to those of other patient groups. The drug was well tolerated at therapeutic plasma levels. These results support the further evaluation of sildenafil as a renoprotective intervention in cardiac surgery.
References:
1. Birnie K et al. Critical Care 2014;18:606.
2. Karkouti K et al. Circulation 2009;119:495-502.
3. Patel NN et al. Heart Fail Rev. 2011;16:553-67.
4. Lledo-Garcia E et al. Transplant Proc 2007;39:1354-6.
5. Patel NN et al. Crit Care Med 2011;39:793-802.
6. Nichols DJ et al. Br J Clin Pharmacol 2002;53 Suppl 1:5S-12S.
7. Vachiery JL et al. Br J Clin Pharmacol 2011;71:289-92.

