| 050P London, UK Pharmacology 2016 |
Sex and agonist-specific mechanisms of cannabinoid tolerance
Introduction: Tolerance to the antinociceptive effects of cannabinoid agonists represents a limitation to their therapeutic potential clinically. Male mice expressing a desensitization-resistant form of the CB1 receptor (S426A/S430A) show delayed tolerance to the hypothermic and antinociceptive effects of Δ9-THC, a strongly desensitizing, partial CB1 agonist [1]. However, it remains unknown whether this mutation can alter tolerance to the synthetic full CB1 agonist CP55,940. Further since tolerance has only previously been examined in male S426A/S430A mutants, it is not known whether tolerance to Δ9-THC and CP55,940 in these mutants differ in a sex-specific manner. Therefore, the purpose of the current study was to determine whether the effects of the S426A/S430A mutation on antinociceptive and/or hypothermic tolerance are sex-specific.
Methods: Tolerance to the hypothermic and antinociceptive effects of the CB1 agonists Δ9-THC (30 mg/kg) and CP55,940 (0.3 and 0.6 mg/kg) were assessed daily for seven days in male and female wild-type and mutant (S426A/S430A) mice. Baseline antinociception and hypothermia were determined immediately prior to and 60 minutes post drug injections (IP). Antinociception was determined using the hotplate assay (%MPE) while hypothermia (%ΔBT) was determined by recording body temperatures. Data were analyzed using two-way repeated measures ANOVAs. Bonferroni’s post hocs tests were used where needed.
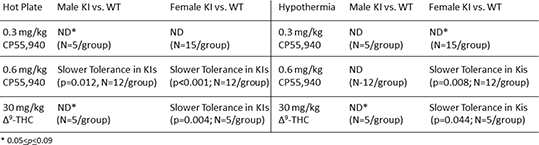
Results: Tolerance developed more slowly to the antinociceptive effects of CP55,940 versus Δ9-THC in both male and female wild-type mice. The S426A/S430A mutation delayed tolerance to the antinociceptive (but not hypothermic) effect of 0.6 mg/kg CP55,940 in male mice (N=12 mice/group). In contrast, the S426A/S430A mutation delayed tolerance to both the antinociceptive and hypothermic effects of 0.6 mg/kg CP55,940 (N=12 mice/group) and 30 mg/kg Δ9-THC in female mice (N=5 mice/group).
Conclusions: Our results demonstrate that sex and agonist specific mechanisms may contribute to cannabinoid tolerance. These studies showcase the antinociceptive therapeutic potential of cannabinoid agonists and offer potential avenues to better understand tolerance to their analgesic effects following prolonged exposure.
References:
1.
Morgan DJ et al. (2014). J Neurosci 34: 5152-5163