Print version
Search Pub Med
| 052P London, UK Pharmacology 2016 |
Natural Killer cells play an important role in placental ischemia induced mitochondrial dysfunction in reduced uterine perfusion pressure (RUPP) rats
Introduction: Preeclampsia (PE), which is characterized by new onset hypertension and is associated with placental oxidative stress.1 Placental ischemia is believed to be the initial event in the development of PE. There is no cure for PE except for the delivery of fetus and the placenta. Mitochondrial dysfunction is a major source of oxidative stress and may play a role in the pathophysiology of PE. We have shown that placental ischemia induces NK cell activation in the reduced uterine perfusion pressure (RUPP) rat model of PE. Thus, we hypothesize that NK cells depletion could decrease oxidative stress and blood pressure in RUPP rats via improving mitochondrial function.
Methods: Pregnant Sprague Dawley rats were divided into three groups; normal pregnant (NP), RUPP, and RUPP+NK cell depletion rats (RUPP+NKD). On gestational day (GD) 14, RUPP surgery was performed, and NK cell depletion with Anti-asialo GM1 antibodies (7µg/100µL, i.p) administered on GD15 &17. On GD19 conscious blood pressure (MAP) was measured, placentas were collected, and mitochondria were isolated to measure mitochondrial function by respiration2 and Complex I activity assays. Decreased rates indicate mitochondrial dysfunction. Respiration measurements (included basal state, state 2, state 3, state 4, and uncoupled states) were obtained using Oxygraph-2k. Complex I activity was measured using the spectrophotometer. Data are expressed as mean ± SEM. One way ANOVA and Bonferroni post hoc test and student t-test were used for statistical analysis.
Results: MAP was elevated in RUPP vs. NP which was normalized in RUPP+NKD (Fig.1). State 3 and uncoupled respiration rates were significantly reduced in RUPP vs. NP, and state 3 was improved in RUPP+NKD (Fig.2). However, there was no change in uncoupled respiration or other respiratory states. Respiratory control ratio (RCR) (state 3/ state 4) was significantly reduced in RUPP vs. NP and significantly improved in RUPP+NKD (Fig.3). Complex I (12 ± 3 vs. 23 ± 2 nmol e-/min/mg, P<0.05) was drastically reduced in RUPP vs. NPs, although Complex I activity increased in RUPP+NKD, it was not significant.
Conclusion: These data indicate that NK cell depletion improved mitochondrial respiration and Complex I activity in response to placental ischemia. Therefore we conclude that NK cells may contribute to oxidative stress in the pathology of PE.
References:
1.Casasco A et al. (1997). Placenta. 18(4):249-53.
2.Lanza IR et al. (2009). Methods Enzymol. 457:349-72.
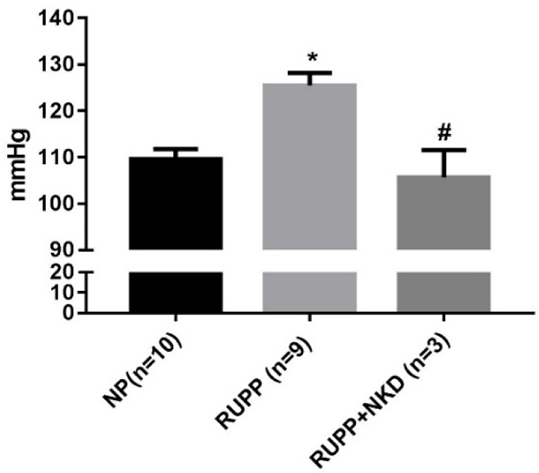
Figure 1. NK cell depletion reduces mean arterial pressure in RUPP rats * p<0.05 vs NP, # p<0.05 vs RUPP
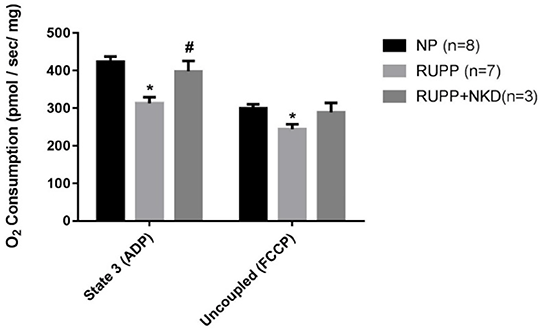
Figure 2. NK cell depletion improves state3 respiration in RUPP placental mitochondria
* p<0.05 vs NP, # p<0.05 vs RUPP
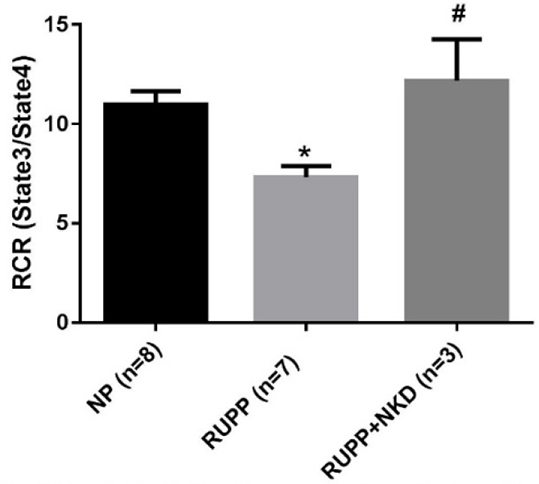
Figure 3. NK cell depletion improves Respiratory Control in RUPP placental mitochondria
* p<0.05 vs NP, # p<0.05 vs RUPP

