Print version
Search Pub Med
| 054P London, UK Pharmacology 2016 |
Anti-viral agent tenofovir induces mitochondrial damage and oxidative stress in hk-2 cells
Introduction: Tenofovir disproxil fumarate (TDF) is a highly effective HIV antiviral drug approved for treating Human Immunodeficiency Virus and Hepatitis B. It is one of the first line drugs used to treat HIV and is efficacious in both novel and treatment-experienced patients. Tenofovir (TFV) is administered orally as the prodrug TDF, which is deesterified to the active drug TFV. However, renal damage is a major adverse effect associated with its use. TFV can induce decreased glomerular filtration rate (GFR), renal failure, and Fanconi Syndrome. The exact mechanism of this toxicity remains unknown, largely due to limited experimental models.
Method: TFV, the active form of TDF, was used for all studies. HK-2 cells were seeded and grown to confluency for 48 h followed by 24-72 h exposure to 0-28.8 uM TFV. The vehicle was phosphate buffered saline (PBS). Cell viability was assessed using the MTT assay and Trypan Blue cell counts. Mitochondrial dysfunction was examined by measuring ATP and ADP levels, evaluating cytochrome c leakage, and by assessing the Cell Energy Phenotype and Bioenergetic Health Index of mitochondria using Seahorse XFp technology1, 2. Oxidative Stress was evaluated by studying protein carbonylation and 4-hydroxynonenal (4-HNE) adducts by Western blot. Data are given as mean±SEM with n=6; analysis was performed using a two-tailed t-test or one-way ANOVA with a 95% confidence interval, p<0.05.
Results: TFV reduced HK-2 cell viability at 24-72 h as shown by the MTT Assay and Trypan Blue exclusion cell counts. TFV reduced ATP levels and increased cytochrome c leakage at 72 h compared to PBS-control. Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) were decreased following 72 h exposure to 28.8 uM TFV. TFV induced oxidative stress. Protein carbonylation and 4-HNE adduct formation were used as biomarkers of oxidative stress; both parameters were increased at 72 h TFV exposure relative to PBS-control.
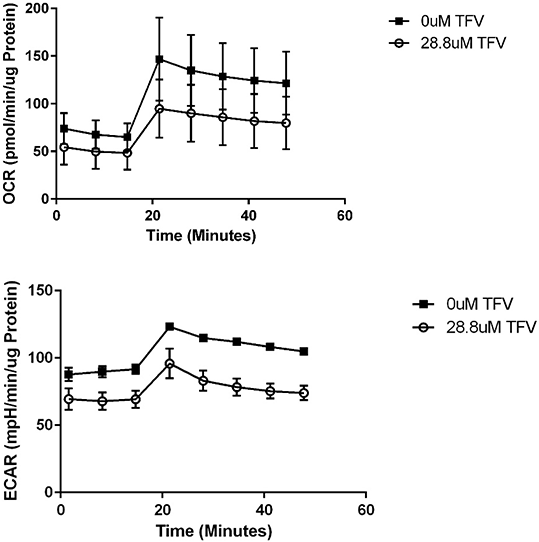
Figure 1 OCR and ECAR following 72 h exposure to 28.8 uM TFV.
Conclusions: TFV cytotoxicity was apparent at clinically relevant concentrations in 24-72 h exposure of HK-2 cells. TFV mediated oxidative stress as indicated by western blot for protein carbonylation and 4-HNE. TFV also induced mitochondrial damage as indicated by decreased ATP, OCR, and ECAR, and cytochrome c leakage.
References:
1. Beeson C et al (2010). J Anal Biochem 404: 75-81.
2. Chacko B et al (2014). Clin Sci 127: 367-373.

