| 068P London, UK Pharmacology 2016 |
Investigating the utility of statins for the prevention of aminoglycoside-induced nephrotoxicity in vivo
Introduction
Megalin-mediated endocytosis is the principal pathway for the accumulation of aminoglycosides in proximal tubule epithelial cells (1), resulting in kidney toxicity. Activation of this pathway depends on intermediates derived from mevalonate, the product of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reduction, catalysed by HMG-CoA reductase (2). We hypothesised that inhibition of HMG-CoA reductase by statins would reduce uptake of aminoglycosides in the proximal tubule, leading to a reduction in toxicity. This has previously been demonstrated in vitro (3). The aim of this study was to test this hypothesis in vivo in two animal models.
Method
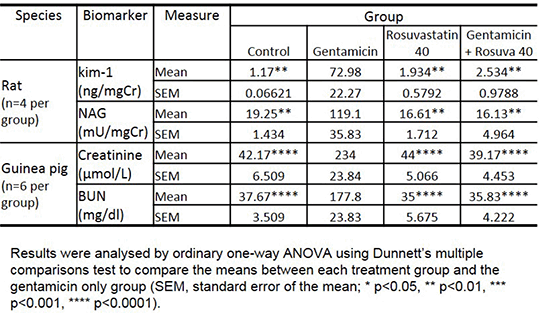
Sprague Dawley rats, (n=4 per group) received intraperitoneal (IP) dosing with saline (control group), gentamicin (200mg/kg/day), rosuvastatin (40mg/kg/day), or gentamicin & rosuvastatin for 9 days. Nephrotoxicity was measured using urinary N-Acetyl-β-D-glucosaminidase (NAG) and kidney injury molecule-1 (kim-1) on urine samples collected within 24 hours after the final dose.
Male Hartley guinea pigs (n=6 per group) received IP dosing with saline (control group), gentamicin (100mg/kg/day), statin, or combined gentamicin and statin (simvastatin or rosuvastatin, 0.4, 4 or 40mg/kg/day) for 9 days. Nephrotoxicity was measured using serum creatinine and blood urea nitrogen (BUN) on blood samples collected 24 hours after the final dose. All procedures met the requirements of the Animals (Scientific Procedures) Act 1986 / ASPA Amendment Regulations 2012.
Results
In rats co-administered rosuvastatin and gentamicin, urinary concentrations of NAG and kim-1 were significantly lower than for gentamicin alone (p<0.01).
In guinea pigs, rosuvastatin reduced gentamicin-induced nephrotoxicity in a dose-dependent manner: doses of 0.4, 4 and 40mg/kg/day led to 46% (p<0.01), 81% (p<0.0001), and 83% (p<0.0001) reductions, respectively, in serum creatinine compared to animals receiving gentamicin only. Similar results were seen with BUN. Rosuvastatin by itself did not affect renal function. Simvastatin did not protect the kidney from gentamicin-induced nephrotoxicity.
Table 1 - Biomarkers of gentamicin-induced kidney toxicity and the impact of rosuvastatin in two animal models.
Conclusions
Rosuvastatin inhibited gentamicin-induced nephrotoxicity in both rat and guinea pig models; in the latter, the effect was seen at therapeutic doses used in man. This work has informed the design of a randomised controlled trial of rosuvastatin for the prevention of aminoglycoside-induced nephrotoxicity in children with cystic fibrosis.
References
1. Schmitz C et al. (2002). J Biol Chem 277: 618-622.
2. Khwaja A et al. (2000). Lancet 355: 741-744.
3. Antoine DJ et al. (2010). Biochem Pharmacol 79: 647-654.

