| 077P London, UK Pharmacology 2016 |
The ‘prophylactic’ and ‘therapeutic’ effects of nadolol in a house dust mite (HDM) -driven murine asthma model
Introduction: Our previous studies have shown that only some β2-adrenoceptor (β2AR) antagonists attenuate the asthma phenotype in an ovalbumin-driven murine model (1). Furthermore, drug treatment began prior to the antigen challenge (prophylactic effect). In the present study we investigated the ‘prophylactic’ and ‘therapeutic’ (effect after phenotype development) effects of the β2AR antagonist nadolol using a more clinically relevant model using house dust mite (HDM) extract.
Methods: Balb/c mice were challenged intranasally with 25 µg HDM protein or saline according to protocols A (prophylactic) and B (therapeutic) (Fig. 1) (2). Nadolol (250 ppm) was mixed with rodent chow and administered orally for 28 days (protocol A) and 33 days (protocol B). The dose was the same as previously used (1). Following the last HDM challenge, mice were euthanized with pentobarbital (100 mg/kg) and evaluated for inflammatory cell infiltration and airway mucus metaplasia (1). Data are expressed as mean ± SEM, and analyzed by one-way ANOVA and Tukey’s post-hoc test.
Results: In the prophylactic model, HDM exposure in mice increased airway eosinophilia (21.16 ± 2.25 vs 0.01 ± 0.001/ml X104, n=6) and mucin volume density (13.60 ± 1.18 vs 0.17 ± 0.04 nl/mm2, n=6) over control mice. This increase was attenuated by chronic nadolol treatment initiated before the phenotype developed (eosinophilia- 7.92 ± 0.81/ml X104; mucin- 4.90 ± 0.57nl/mm2, n=5). In the therapeutic model, the initial HDM exposure increased airway eosinophilia (9.57 ± 0.95 vs 0.01 ± 0.001/ml X104, n=8) and mucin volume density (12.71 ± 0.49 vs 0.07 ± 0.004 nl/mm2, n=8) over control mice, which were completely resolved after 4 weeks. Subsequent re-exposure to HDM restored the increase in both eosinophilia (9.48 ± 0.89 vs 0.02 ± 0.002/ml X104, n=7) and mucin volume density (13.27 ± 0.73 vs 0.83 ± 0.20 nl/mm2, n=8) over the resolved group. Chronic nadolol treatment following the initial HDM exposure decreased eosinophilia (3.22 ± 0.60 vs 9.48 ± 0.89/ml X104, n=8) and mucus secretion (6.65 ± 0.44 vs 13.27 ± 0.73 nl/mm2, n=6) in response to HDM re-exposure.
Conclusion: Our results suggest that chronic nadolol treatment attenuates the asthma phenotype in murine asthma models, when administered both pre- and post-development of the phenotype. These studies corroborate our previous findings in the ovalbumin-driven model and provide further evidence for the therapeutic efficacy of nadolol in asthma.
References:
1 Thanawala VT et al. (2015). Br J Pharmacol. 172:4833-46
2 Cates EC et al. (2004). J Immunol. 173:6384-6392
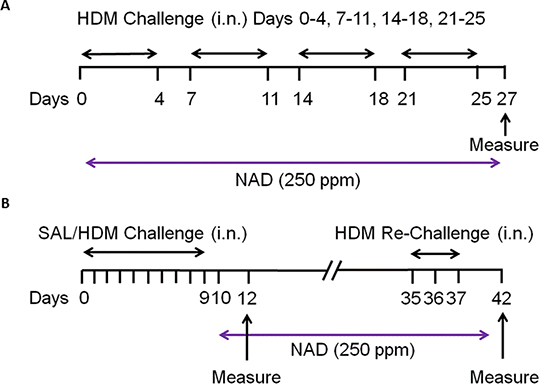
Fig. 1 Protocol Timelines for the 'Prophylactic'(panel A) and 'Therapeutic'(panel B) asthma models

