| 103P London, UK Pharmacology 2016 |
Endogenous allosteric modulators of G protein-coupled receptors - unappreciated mediators in health and disease?
Introduction: Synthetic allosteric modulators of G protein-coupled receptors (GPCRs) now have validated pharmacological and therapeutical potential. However, the fact that most (if not all) GPCRs possess allosteric sites begs the question as to whether some of the sites of action of such molecules represent unappreciated ‘orphan’ allosteric sites for hitherto unidentified natural modulators (1). One example is major basic protein (MBP), previously implicated in asthma symptomatology via antagonism of neuronal autoinhibitory M2 muscarinic receptors (mAChRs) in the airways (2). One obvious structural feature of MBP is its high content in arginine residues, rendering it highly basic (pI~10). We hypothesised that, beyond MBP, other basic, arginine-rich endogenous proteins can interact allosterically with the M2 mAChR. The aim of this study was to identify and characterise novel putative endogenous allosteric modulators at the M2 mAChR.
Methods: Using CHO cells stably expressing mAChRs, we performed [3H]NMS radioligand binding and [35S]GTPγS binding functional studies to assess the allosteric effects of highly basic endogenous peptides co-localised with the M2 mAChR, including: MBP, protamine, apelin-36 and LL-37. Data are presented as mean ± SEM and statistical analysis on affinity and cooperativity parameters of the modulators was performed using one-way ANOVA or Student’s t-test, as applicable.
Results: All of the selected endogenous peptides showed partial inhibition of [3H]NMS specific binding in the micromolar range, with negative co-operativity with the radioligand. Additionally, these peptides significantly altered [3H]NMS dissociation from the orthosteric site, a hallmark of allosteric mode of binding. Importantly, all investigated peptides negatively modulated the functional properties of the endogenous orthosteric agonist, ACh (Table 1).
Table 1. Summary table of pharmacological properties of basic peptides at the M2 mAChRs
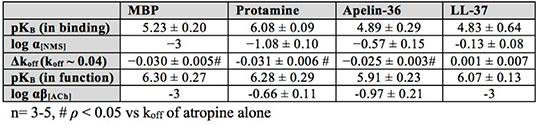
Conclusion: Our results highlight that, at least several endogenous peptides, known to be co-localised with the M2 mAChR, can bind allosterically and modulate ACh function. This suggests that, in the context of disease such as infection and inflammation, where MBP and LL-37 are found at their highest concentrations (~10-30uM), these peptides will block the activity of the M2 mAChR, potentially worsening the diseases. Beyond the scope of our study, we anticipate that the role of physiologically expressed allosteric modulators can be applied to other GPCRs and will have a major impact on modern drug discovery.
References: 1. van der Westhuizen ET et al. (2015). J Pharm Exp Ther 353(2):246-60. 2. Jacoby DB et al. (1993). J Clin Invest 91:1314-1318

