| 105P London, UK Pharmacology 2016 |
Role of peroxisome proliferator-activated receptors-alpha (PPARɑ) and the transient receptor potential vanilloid receptor (TRPV1) in mediating the anti-nociceptive effects of palmitoylethanolamine in rat
Introduction: N-palmitoylethanolamine (PEA) is a ligand at peroxisome proliferator-activated receptors-alpha (PPARÉ‘), a nuclear receptor which has anti-inflammatory effects (1). Herein, Complete Freund’s adjuvant (CFA)-induced inflammatory pain model in rat and in vitro calcium imaging studies were used to evaluate the mechanisms that underlie the anti-nociceptive effects of PEA.
Methods: Adult male Sprague Dawley rats (180-200g) received subcutaneous injections of CFA (0.1 ml) or saline into the plantar surface of the left hindpaw. Von Frey filaments were applied to the mid-plantar aspect of the left hind paw using the “up-down” method to determine the paw withdrawal threshold (PWT). PEA (50 µg), WY14643 (50 µg, a selective PPARα agonist), PEA (50 µg) plus GW6471 (50 µg, a selective PPARα antagonist) or WY14643 (50 µg) plus GW6471 (50 µg) were injected into the plantar surface of the left hindpaw at day 7 post-CFA injection, then the behavioral tests were repeated 6 hour post-drug administration. DRG neurones were prepared for calcium imaging (2). Neurones were loaded with Fura 2AM. Capsaicin (15nM)-evoked changes in [Ca2+]i and the effects of 6 hours pre-incubation of 30µM PEA on capsaicin-evoked calcium responses were determined. Changes in [Ca2+]i were measured as ratios of peak florescence at excitation wavelengths of 340nm and 380nm and expressed as a percentage of the KCl (60mM) response. Statistical analysis used Student’s t test or one way ANOVA test followed by Dunnett’s post-hoc as appropriate.
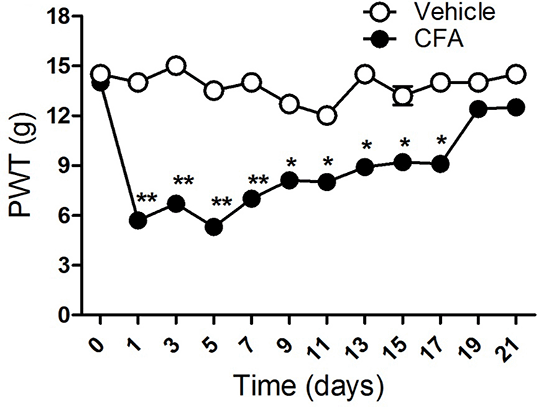
Results: PWTs were significantly reduced 1 day after CFA injection. This reduction persisted for about three weeks (Fig 1). Both PEA and WY14643 significantly restored PWT (3.8 ± 0.24 vs 8.4 ± 1.2, n = 6 rats, P<0.01) and (3.81 ± 0.25 vs 8.42 ± 1.18, n = 6 rats, P<0.01) respectively in a PPARα-dependent fashion (8.4 ± 1.2 vs 5.33 ± 1.19, n = 6 rats, P<0.01) and (8.42 ± 1.18 vs 5.27 ±0.47, n = 6 rats, P<0.01). 15nM capsaicin produced 63.9 ± 13.4 % of KCl response (n = 68 neurons). Pre-incubation of DRG neurones with PEA 6 hours prior to stimulation with capsaicin, significantly reduce capsaicin-evoked calcium responses (42.9 ± 6.4 % of KCl response, n=54, P<0.001.
Conclusions: Modulating the activity of the TRPV1 channel could provide the mechanism that underlies the PPARα-mediated anti-nociceptive effects of PEA.
References
1. LoVerme J et al. (2006). J Pharmacol Exp Ther 319: 1051-1061
2. Millns PJ et al. (2001). Br J Pharmacol 132: 969-971