| 125P London, UK Pharmacology 2016 |
Modulating PAR2 signalling by novel compounds based on GB88
Introduction: Proteinase-activated receptor-2 (PAR2) is a G protein-coupled receptor (GPCR) that we have previously shown to play a key role in mediating chronic joint inflammation 1. A novel small molecule PAR2 antagonist called GB88 has recently been developed 2 with promising data showing inhibition of PAR2-mediated pain and inflammation in vivo3. Here, we explore the pharmacology of non-peptide compounds that were originally based on the structure of GB88.
Method: Characterisation of GB88-related compounds was carried out in HEK293 cells (endogenously expressing PAR2) and a PAR2 NFκB-reporter cells (NCTC2544-derived). All compounds were synthesised in house and screened for modulation of PAR2 function across an array of assays. Compounds were first screened in an NFκB-reporter assay where modulation of PAR2-mediated NFκB transcriptional activity was assessed4. The same compounds were also screened for PAR2-induced calcium mobilisation (Fluo4-AM, FlexstationIII) and the activation of ERK MAP kinase phosphorylation by Western blotting.
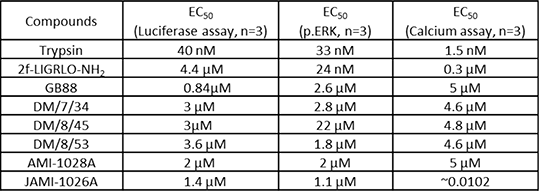
Results: Five novel compounds; DM/7/34, DM/8/45, DM/8/53, JAMI-1028A and JAMI-1026A were tested alongside GB88 5. All compounds, including GB88, stimulated to an increase in NFκB transcriptional activity when tested alone. The GB88 response was comparable (6.720 ± 0.391 fold, n=3) to 2f-LIGRLO-NH2 (8.390 ± 0.812 fold, n=3). However, pre-treatment of cells with either GB88 or the GB88-derived compounds did not inhibit PAR2 activity mediated by 2f-LIGRLO-NH2 or trypsin. Furthermore, all compounds displayed a concentration-dependent phosphorylation of ERK. The same compounds were also evaluated using Ca+2 assays and demonstrated a concentration-dependent increase in intracellular Ca+2 mobilisation. EC50values obtained for each compound are shown in Table 1.
Conclusions: All the compounds, including the proposed PAR2 antagonist GB88, displayed agonist activity albeit less potent than trypsin or 2f-LIGRLO-NH2. No inhibition of PAR2 activity was observed for any of the compounds tested in this study. Further work is required to explore their pharmacological properties in other assays and in response to other PAR2 agonists.
References:
(1) McIntosh KA et al. (2007) Curr Opin Pharmacol. 7(3):334-8. (2)Suen et al. (2012) Br J Pharmacol. 165(5):1413-23; (2014) Br J Pharmacol Sep; 171(17):4112-24.(3) Lieu T et al. (2016) Br J Pharmacol; 173: 2752-2765.(4) Kanke et al. (2009), Br J Pharmacology 158: 361-371.(5) Barry et al. (2010), J.Med. Chem 53: 7428-7440.
Table 1: EC50 values for the six compounds compared with trypsin and 2f-LIGRLO-NH2