| 137P London, UK Pharmacology 2016 |
Characterisation of drug-like inhibitors of deadenylase enzymes by AMP detection and differential scanning fluorimetry
Introduction:
In eukaryotes, the removal of the poly (A) tail of cytoplasmic mRNA (deadenylation) is a crucial step in post-transcriptional gene regulation. The main enzymes involved in regulated mRNA deadenylation are the Caf1 and Ccr4 ribonucleases whose activities are attributed to the DEDD (Asp-Glu-Asp-Asp) or the EEP (endonuclease-phosphatase) domain respectively. Two magnesium ions are required at the active site for activity1. These enzymes are possible drug targets in diseases such as metastatic cancer, osteoporosis, bone repair and obesity. To facilitate the discovery, development, and characterisation of small drug-like inhibitors of these enzymes, we recently developed a sensitive fluorescence-based assay for deadenylase activity based on detection using an oligonucleotide probe complementary to the RNA oligonucleotide substrate2. The aim of this study was to develop more biochemical assays useful for characterisation of molecular inhibitors.
Method:
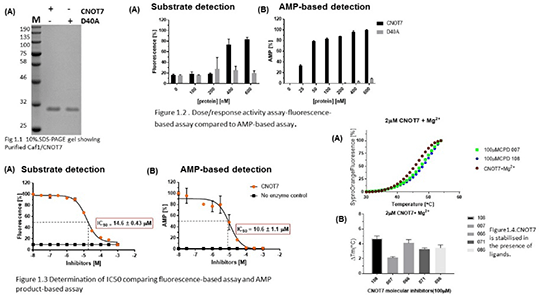
First, we expressed human Caf1/CNOT7 enzyme in bacteria cells and purified to homogeneity using His-trap affinity chromatography. Next, we used a chemiluminescence-based detection assay of AMP, a reaction product of deadenylase enzymes to determine enzyme activity as a function of concentration in comparison with recently developed fluorescence-based assay. Inhibitory Concentrations (ICâ‚…â‚€) of drug-like molecules of Caf1/CNOT7 was also determined. In addition, differential scanning fluorimetry (DSF) also known as thermal shift assay was evaluated as a means to characterise binding of compounds to Caf1/CNOT7 enzyme. ICâ‚…â‚€ and thermal shift data are given as mean ± SEM (n=3).
Results
The results indicate that a minimal amount of enzyme is required for assays using the AMP detection when compared to the fluorescence-based assay which requires a ten-fold amount of enzyme. The IC50 values determined comparing both methods were shown to be in a similar range. In addition, thermal shift assays showed enzyme inhibition by a shift in curve in the presence of the inhibitors.
Conclusion
The AMP detection is a highly sensitive assay with low background/noise ratio that can be used as an alternative to the previously developed fluorescence-based assay for screening of compound libraries and the determination of IC50 values. Also, the DSF is a useful assay to determine the binding mode of inhibitory compounds. These assays complement existing tools available for the characterisation of drug-like inhibitors of deadenylase enzymes.
References
1. Winkler and Balacco, 2013.
2 Maryati et al, 2014.