Print version
Search Pub Med
| 149P London, UK Pharmacology 2016 |
The impact of P450 oxidoreductase knock out on systemic exposure to rosuvastatin, atorvastatin and atorvastatin metabolites
Introduction
Statins are amongst the most commonly prescribed medications worldwide. Although generally well tolerated, myotoxicity attributable to statin therapy occurs in ~5% of patients. Atorvastatin (ATV) is hydroxylated by CYP3A4; concomitant use of CYP3A4-inhibiting drugs is an established ATV myotoxicity risk factor. Rosuvastatin (RVT) is metabolised only to a minor extent by CYP2C9 (~10%). ATV and RVT are also substrates for phase II enzymes and transporters. P450 oxidoreductase (POR) is the major electron donor for all microsomal CYP enzymes; importantly, its effects on statin pharmacokinetics are unknown. This work aimed to determine whether POR deficiency alters statin exposure using the selective hepatic POR null murine model (HRN).
Method
1.) Hepatic microsomes from three wild-type (WT) and three HRN male mice were incubated (200μL final volume) for: a) 30 minutes with ATV (1-250μM) and; b) up to 120 minutes (ATV) or 240 minutes (RVT) at 20μM.
2.) Separately, three WT and three HRN male mice each received 30mg/Kg ATV and 30mg/Kg RVT together via intraperitoneal injection (10mL/Kg). Serial blood samples were collected up to 24 hours onto dried blood spots. The study was carried out in accordance with the Animal Scientific Procedures Act of 1986 and after a local ethics review.
All bioanalysis was by liquid chromatography-mass spectrometry (Sciex 6500) using a method validated according to the FDA guidelines (2001). Pharmacokinetic analysis used the Real Statistics Resource Pack Excel add-in; statistical analysis was by Student’s t-test (p<0.05 significant)
Results
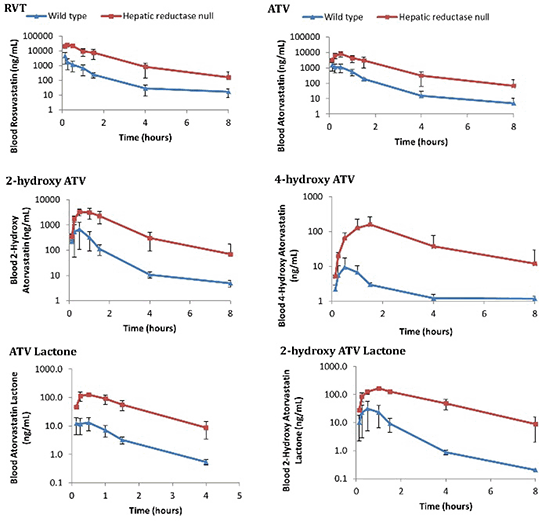
The in vitro microsome incubations demonstrated that POR deficiency is associated with decreased ATV hydroxylation (Figure 1), but had no effect on RVT (Figure 2). Unexpectedly, there was a significant increase in the in vivo blood maximum concentrations and exposures of ATV, all ATV metabolites and RVT in HRN mice (Figure 3).
Figure 1 Hepatic microsomal POR-dependent ATV hydroxylation
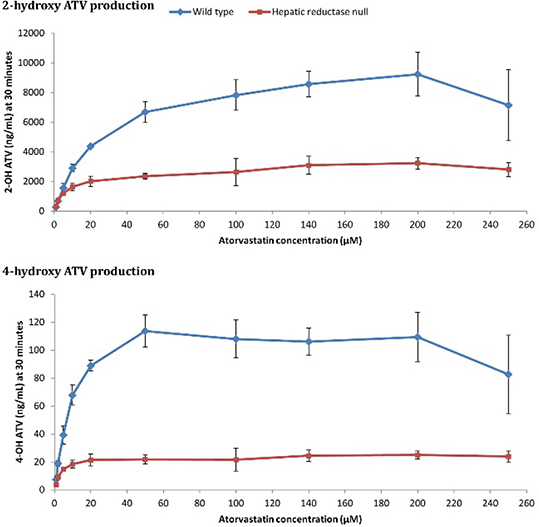
Figure 2 Proportions of 20μM ATV and RVT remaining in microsomal incubations
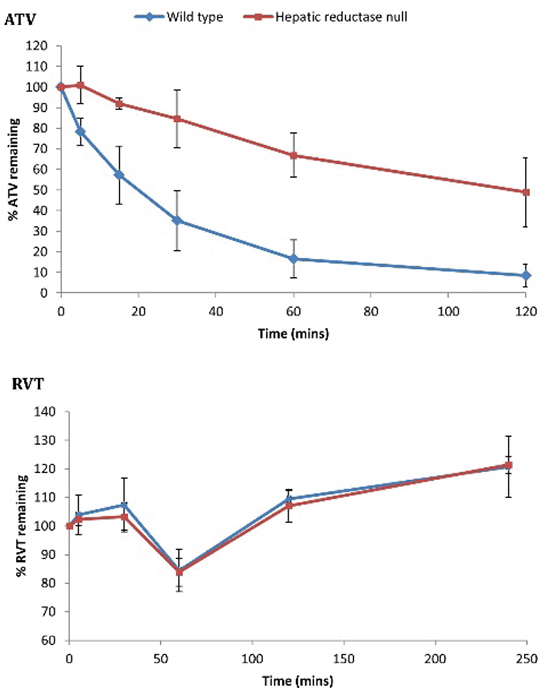
Figure 3 In vivo pharmacokinetic profiles
Conclusions
These results demonstrate that POR deficiency is associated with reduced ATV hydroxylation in vitro, reflecting decreased CYP3A activity. The in vivo increase of ATV lactone may represent a compensatory increase in non-POR dependent UDP-glucuronyltransferase activity in HRN livers. The unexpected increase of hydroxy-ATV metabolites and RVT in HRN mice in vivo suggests extra-hepatic CYP3A-mediated hydroxylation, an effect of transporters or potentially reduced hepatic uptake due to the fatty liver that develops in HRN mice. Further research to characterise HRN transporter protein expression is ongoing.

