| 173P London, UK Pharmacology 2016 |
Characterisation of the signalling mechanisms involved in the induction of cyclooxygenase-2 by interleukin-4
Introduction: Historically cyclooxygenase (COX)-2 has been associated with the pro-inflammatory phase of inflammation; recently there is evidence to suggest the protein also has an anti-inflammatory role. A number of research groups have given in vivo evidence showing inhibition of COX-2 in the late stages of the inflammatory response to exacerbate inflammation suggesting COX-2 to also play a pro-resolution role1. If proven, this phenomena may open new doors for research as this late phase COX-2 could be considered a target for the early resolution of inflammation. In comparison to lipopolysaccharide (LPS)-stimulated macrophages, we showed diclofenac induced COX-2 was associated with anti-inflammatory cytokine release and required activation of peroxisome proliferator-activated receptor-γ (PPAR-γ)2. In the current study, we sought to investigate a potential endogenous inducer for the PPAR-γ-dependent COX-2 enzyme and to elucidate the molecular pathway associated with induction of this protein.
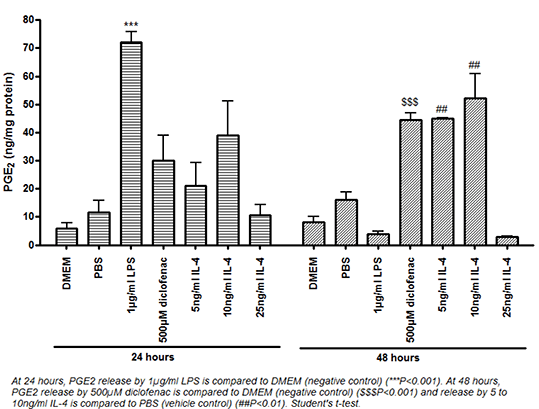
Methods: J774.2 mouse macrophages were stimulated with 1µg/ml LPS, 500µM diclofenac or 10ng/ml of the anti-inflammatory cytokine, interleukin-4 (IL-4) for either 24h or 48h. Samples were analysed via Western blotting and enzyme immunoassay for COX-2 expression and activity, respectively. To determine whether PPAR-γ is required for IL-4-induced COX-2, cells were stimulated with IL-4 in the presence or absence of the PPAR-γ antagonist, Bisphenol A diglycidyl ether (BADGE). Expression of phosphorylated p38 mitogen-activated protein kinase was also determined under similar experimental conditions as p38 has previously been linked to COX-2 expression.
Results: IL-4 [5-100ng/ml] induced a catalytically active COX-2 protein. At 48h, the induction of COX-2 by 10ng/ml IL-4 was blocked by BADGE (Figure 1, upper panel) with no effect on phospho-p38 expression (figure 1, lower panel). Additionally, inhibition of p38 with 1-100µM SB203580 blocked induction of COX-2 by 10ng/ml IL-4 at 48h.
Conclusion: In macrophages, IL-4 is able to induce a catalytically active COX-2 protein in a PPAR-γ-dependent manner at 48h which requires p38. PPAR-γ is typically associated with anti-inflammatory pathways and this suggests induction of a COX-2 protein associated with macrophages polarised to an anti-inflammatory phenotype. Preliminary evidence has shown the diclofenac and IL-4 treated macrophages to possess anti-inflammatory phenotypic characteristics compared to the LPS treated cells as shown by increased cell migration in wound healing assays; a phenotype associated with anti-inflammatory macrophages3.
References: (1) Gilroy DW et al. (1999). Nat Med, 5: 698-701. (2) Ayoub SS et al. (2009). Mol Cell Biochem, 327: 101-10. (3) Kapellos TS and Iqbal AJ (2016). Mediators of Inflammation 2016: Epub 10th April 2016.