| 176P London, UK Pharmacology 2016 |
The role of PP2A and related proteins in Human Brain Microvascular Endothelial Cells in response to Pro and Anti-Inflammatory Cytokines
Introduction
The endothelial layer of the microvasculature is both a physical and metabolic barrier between the peripheral blood and the brain. Disruption to this protective wall has been linked to diseases such as Alzheimers Disease and Multiple Sclerosis. PP2A being the most prevalent phosphatase in the brain and little is known about its role. The aim of this study is to: 1) investigate the effect of pro and anti-inflammatory cytokines on vascular permeability. 2) determine if these cytokines modulate PP2A, CIP2A, MTOR and c-JUN expression.
Method
Human brain microvascular endothelial cells (HBMECs) were cultured in serum free media and treated with IL-13 (20ng/ml), IL-4 (20ng/ml), LPS (100ng/ml), IFN-γ (20ng/ml) and a combination of IL-4 + IL-13 (anti-inflammatory) and LPS and IFN-γ (pro-inflammatory). Cells were seeded onto a porous membrane. Transendothelial movement of FITC-dextran was measured over 24h to reflect permeability compared to the non-treated control. Data are represented as area under the curve. The mRNA expression profile of the aforementioned genes were analysed by real-time RT-PCR and expressed as fold increase following normalization to GAPDH and GPI. Data are presented as mean±S.E.M. (n=4) and analysed by one-way ANOVA with post hoc (Bonferroni). P<0.05 indicates statistical significance (*).
Results
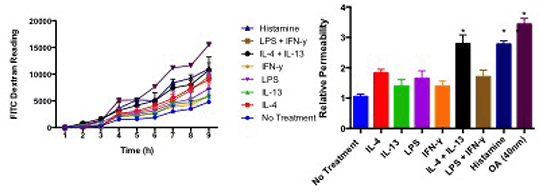
Fig. 1 Relative permeability of HBMECs treated with inflammatory cytokines.
| IL-4 (20ng/ml) | IL-13 (20ng/ml) | LPS (100ng/ml) | IFN-γ (20ng/ml) | IL-4 (20ng/ml) + IL-13 (20ng/ml) | LPS (100ng/ml)+ IFN-γ (20ng/ml) | ||
| PP2CA | -47.1.1±9.5%* | -40.1±15.9% | -58.1±10.6%* | -87.9±3.1%* | -49.6±14.2% | -80.9±6.5%* | |
| CIP2A | -62.35±12%* | 7.6±6.7% | -53.3±9.3%* | -71.1±10%* | -60.3±3.2%* | -73.3±8.3%* | |
| C-MYC | -9.4±14.38% | -17.6±7.8% | -34.3±18.5% | -14.3±23.0% | -19.3±23.2% | ||
| C-JUN | -78.5±8.0%* | -21.1±1.8%* | -34.1±9.9 | -5.8±16.5% | -54.4±12.1% | -43.8±3.1%* | |
| MTOR | -36.7±10.4% | -10.0±6.0% | -62.7±23.0% | -69.3±16.3%* | -24.0±19.1% | -53.5±15.2% | |
Table 1 Effect of inflammatory cytokines on PP2A, CIP2A, c-MYC, c-JUN and MTOR RNA expression in HBMEC.
IL-4 alone and in combination with IL-13 increased HBMEC permeability (Fig. 1) as did okadaic acid, a PP2A inhibitor (3.4±0.2 fold; P<0.05) compared to control. Moreover, cytokines differentially modulated mRNA expression of transcripts associated with PP2A signaling (Table 1).
Conclusion
In conclusion, anti-inflammatory cytokines increase permeability in HMECs, an effect mimicked by inhibition of PP2A. Therefore, targeting PP2A may be a potential therapeutic target to prevent transendothelial migration.
1. Price et al, Curr Opin Neurobiol. 1999;9(3):336–42.

