Print version
Search Pub Med
| 182P London, UK Pharmacology 2016 |
Pharmacological inhibition of NLRP3 inflammasome exerts beneficial effects against ischaemia/reperfusion injury in the rat heart by local improvement of protective signaling pathways
Introduction: The NLRP3 inflammasome is a large multimeric danger-sensing platform that promotes activation of caspase-1 and mediates the cleavage of inactive pro-IL-1β into its active form [1]. We and others have recently documented its central role in the inflammatory response to tissue injury [2,3]. Besides, we have shown that the NLRP3 inflammasome activation amplifies myocardial ischemic injury in diabetic mice [4]. Here, we investigate whether the pharmacological modulation of NLRP3 inflammasome with a small electrophilic molecule, INF-4E [5], exerts protective effects in an ex-vivo model of myocardial ischaemia/reperfusion (IR) injury.
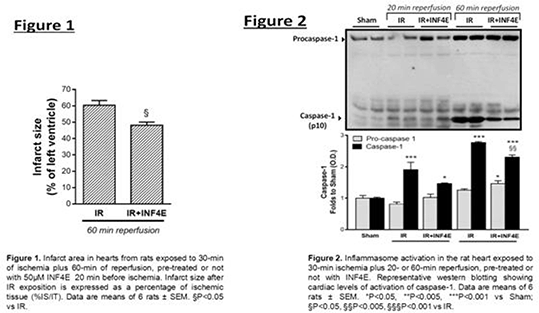
Method: Isolated hearts from 20 male Wistar rats (European Directive 2010/63/EU, approved by local ‘Animal Use and Care Committee’) underwent perfusion without ischaemia (Sham) or IR (30 min ischemia plus 20 or 60 min reperfusion) with and without INF-4E treatment (50μM for 20 min before ischaemia). Infarct areas were assessed at the end of the 60 min reperfusion with the nitro-blue-tetrazolium (NBT) technique and left ventricular pressure was recorded as index of contractile activity throughout the experiment. Immunoblot analyses were performed to assess Tyr204ERK1/2, Ser473Akt, and Ser9GSK-3β phosphorylation and NLRP3, caspase 1, mtTFA and NRF1 expression. All values are expressed as means±SEM and were analyzed by ANOVA test followed by Bonferroni\'s post-test. A p<0.05 was considered statistically significant.
Results: Administration of INF-4E attenuated myocardial IR, as shown by a significant reduction in infarct size (60%vs.48% %IS/IT, Figure1) and improvement in left ventricular pressure. Western blot analysis demonstrated that IR injury evoked the formation of the NLRP3 inflammasome complex. Interestingly, INF-4E treatment attenuated the IR-induced increase in caspase-1 activity (p<0.005, Figure.2) thus confirming the drug’s ability to affect the NLRP3 inflammasome activation. The hearts of INF4E-pretreated animals displayed a marked activation of the protective RISK pathway (increased phosphorylation of Tyr204ERK1/2, Ser473Akt, and Ser9GSK-3β). This effect was associated with an increased expression of markers of mitochondrial oxidative phosphorylation (mtTFA and NRF1).
Conclusion: The results demonstrate that the pharmacological inhibition of the NLRP3 inflammasome complex was associated with significant reduction in myocardial IR injury. These beneficial effects were due, at least in part, to a robust improvement of the activity of important intracellular protective pathways within the rat heart.
References:
1. Benetti E et al., (2013). Mediators.Inflamm. 2013:1-12.
2. Strowig T et al., (2012). Nature. 481:278-286.
3. Chiazza F et al., (2015) Mol.Med. 21:1025-1037
4. Mastrocola R. et al., (2016). Oxid.Med.Cell.Longev. 2016:3480637
5. Cocco M et al., (2014). J.Med.Chem. 57:10366-82.