| 190P London, UK Pharmacology 2016 |
The effects of fluoxetine exposure during pregnancy in the rat
Introduction: SSRI antidepressants (particularly fluoxetine) are the most commonly prescribed psychotropic drug in pregnancy1. Although SSRIs cross the placental barrier, there is a lack of safety data regarding the effects of SSRI exposure in utero. The aim of this study was to investigate fluoxetine during pregnancy in rats, utilizing a clinically relevant treatment regime and scenario with doses in the pharmacological range, an approach used previously in our laboratory for methamphetamine2.
Methods: Female Sprague-Dawley rats (approx. 4 months old) were mated and singly housed once producing a smear positive for sperm. From gestational day (GD) 7 until littering, rats received either vehicle, 5, 10 or 20 mg/kg fluoxetine (FLX) via oral gavage (n=13-15/group). Upon littering, litter characteristics were recorded, such as the number of pups born live. Data was analysed using a One-way or Two-way ANOVA, followed where appropriate by post hoc SNK test. Pup mortality data was analysed using a Chi-Squared test; p<0.05 was deemed statistically significant.
Results: Body weight gain was significantly reduced in the 10 and 20 mg/kg FLX treated groups (Figure 1A). Food consumption was significantly reduced during the first week of dosing in the 10 and 20 mg/kg FLX groups and in the second week with 20 mg/kg only; water consumption was reduced in both weeks of dosing in the 20 mg/kg FLX group. A significant reduction in offspring birth weight was associated with the 20 mg/kg FLX group (Figure 1B). There was a dose-related increase in the numbers of stillborn pups, which was significantly different at all dose levels when compared to controls.
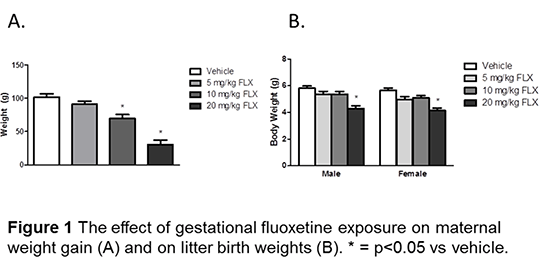
Conclusions: Fluoxetine causes a significant effect on maternal weight gain during pregnancy at the two higher doses employed. Moreover, the mortality rate at littering was increased at all doses. Overall the results indicate that fluoxetine exposure during pregnancy to rats even at doses in the pharmacological range has a considerable effect on both mothers and pups. Such findings do suggest that fluoxetine exposure during pregnancy does pose risks if extrapolated to the clinical scenario, and whether such patterns are evident in other marketed SSRIs will be the source of future work.
References:
1. Alwan S et al. (2016). CNS Drugs. 30: 499-515.
2. McDonnell-Dowling K et al. (2014). Int J Dev Neurosci 35: 42-51
Acknowledgement: This work was supported by a College of Science, NUI Galway postgraduate fellowship.

