Print version
Search Pub Med
| 192P London, UK Pharmacology 2016 |
Pharmacokinetic/pharmacodynamic target attainment of co-amoxiclav and piperacillin-tazobactam in an adult intensive care unit
Introduction Treatment of infection in intensive care (ICU) commonly involves the use of beta-lactam antibiotics piperacillin-tazobactam or co-amoxiclav. Efficacy of beta-lactam antibiotics is dependent upon the pharmacokinetic/pharmacodynamic (PK/PD) target of time above minimum inhibitory concentration (MIC) of the target organism. We measured antimicrobial concentrations in patients receiving these antibiotics on the adult ICU to see how many were achieving PK/PD targets.
Methods This was a subgroup, interim analysis of an ongoing clinical PK study, ABDose, which was completed for a student BSc project. Participants were adults in ICU receiving antibiotics as part of their care. Informed consent was obtained. In our unit, piperacillin-tazobactam is given as a prolonged (four-hour) infusion and co-amoxiclav as an intermittent (five-minute) bolus. Demographics and measures of organ function were recorded. Plasma antibiotic concentrations were measured, at steady-state, at 50% and 100% of the dosing interval. We chose PK/PD targets of antibiotic concentration above MIC and a more conservative 4x above MIC of likely pathogen or microbiological isolate (when available). These targets have been used previously [1]. MIC was taken from the European committee on antimicrobial susceptibility testing (EUCAST) [2].
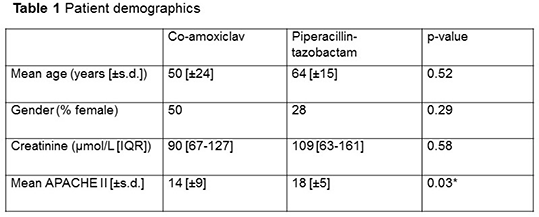
Results 32 participants received piperacillin-tazobactam (n=14) or co-amoxiclav (n=18). Participant demographics are displayed in Table 1. At 50% of the dosing interval, 69%(n=11) of those receiving co-amoxiclav achieved plasma concentrations >MIC compared to 100%(n=11) of those receiving piperacillin-tazobactam (p=0.06); the PK/PD target of >4xMIC was reached by 38%(n=6) of those receiving co-amoxiclav compared to 100%(n=11) of those receiving piperacillin-tazobactam (p=0.001*). At 100% of the dosing interval, 50%(n=5) of those receiving co-amoxiclav achieved plasma concentrations >MIC compared to 89%(n=8) of those receiving piperacillin-tazobactam (p=0.141) and 20%(n=2) of co-amoxiclav participants achieved >4xMIC compared to 56%(n=5) of piperacillin-tazobactam participants (p=0.17).
Conclusions In this interim dataset, a significant proportion of patients failed to achieve PK/PD targets. PK/PD target attainment was less likely in patients receiving bolus dose co-amoxiclav compared to prolonged infusion of piperacillin-tazobactam. These interim findings support the need for further work to investigate optimal beta-lactam dosing strategies. Population-PK modelling will be undertaken on the final ABDose dataset to help identify optimum dosing regimens.
References 1. Roberts J et al. (2014). Clinical Infectious Diseases 58 (8):1072-1083. 2. EUCAST clinical breakpoints, http://www.eucast.org/clinical_breakpoints/