Print version
Search Pub Med
| 193P London, UK Pharmacology 2016 |
A population pharmacokinetic model of co-amoxiclav in adult intensive care patients
Introduction: Co-amoxiclav is commonly prescribed for patients in UK intensive care units (ICUs), for the treatment and prevention of a wide range of infections. Efficacy of beta-lactam antibiotics like amoxicillin is dependent upon the time spent above minimum inhibitory concentration (MIC) of the target organism. A previous pharmacokinetic/pharmacodynamics (PK/PD) study of co-amoxiclav suggested that 6-hourly dosing is appropriate for most patients(1). In the UK, 8-hrly dosing is standard and recent observational data suggests a high proportion of critically ill patients fail to achieve antimicrobial PK/PD targets(2) .
ABDose is an ongoing observational population-PK/PD study of common antimicrobials used in ICU. This interim analysis aimed to investigate the PK/PD of amoxicillin in the first cohort of participants prescribed co-amoxiclav.
Methods: Participants were adults in ICU receiving intravenous co-amoxiclav as part of standard treatment. Following informed consent/assent, blood samples were obtained at predefined time-points between doses. Plasma was frozen at -80°C and concentrations measured using high-performance liquid chromatography/mass spectrometry. Population-PK modelling was undertaken using non-linear mixed-effects modelling software (NONMEM v7.3, Icon plc). Previously suggested minimum PK/PD targets of concentration above MIC at 50% and 100% of the dosing interval were used, alongside more conservative targets of concentration >4xMIC(2).
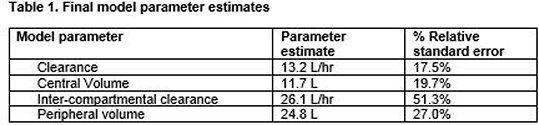
Results: In this interim analysis, 151 samples were available from 22 participants. Demographic/clinical characteristics included age 59±22 years (mean±standard deviation), weight 80.8±17.2 kg and serum creatinine 72-80 µmol/L (median [interquartile range]). A two-compartment structural model with inter-subject variability on the central compartment\'s volume of distribution and clearance provided the best model fit. Additional covariate analysis (weight/creatinine) found serum creatinine to have a significant effect on clearance in the model (p=0.05). Final structural PK parameter estimates are provided in table 1.
17 participants had concentrations available at time-points for analysis of PK/PD target attainment. At 50% of the dosing interval 13(76%) participants had concentrations >MIC, only 6(35%) of these had concentrations >4xMIC. At 100% of the dosing interval these figures were 8(47%) participants >MIC and 2(12%) >4xMIC.
Conclusions: A significant proportion of patients in ICU fail to achieve even minimum PK/PD targets. A two-compartment structural population-PK model with inter-subject variability, as described above, provided the best fit for this interim amoxicillin data. Future work will include full covariate analysis, including paediatric data, clavulanate concentrations and simulation of optimal dosing strategies.
References:
1. Carlier M et al. (2013). J. Antimicrob. Chemother 68 (11):2600-2608
2. Roberts J et al. (2014). Clinical Infectious Diseases 58 (8):1072-1083.