| 199P London, UK Pharmacology 2016 |
Comparison of the direct and indirect vascular actions phenylephrine and pseudoephedrine, as orally-active nasal decongestants, with reference to plasma concentrations found in man
Introduction: Since 2006 phenylephrine (PHE), a selective α1-adrenoceptors agonist has been used in the UK as a decongestant ingredient. However, there is little clinical data to support its use1 and PHE capsules (12mg) were no better than placebo at improving nasal airflow2. Pharmacokinetic data in man found that oral PHE can only achieve 10-30nM in the plasma3. Thus, we have compared the actions of PHE, with that of a pseudoephedrine (PSE), in the porcine splenic artery (PSA). Oral PSE (60mg) is known to achieve a peak concentration of 1-3µM4.
Methods: The PSA was dissected and prepared for isometric tension recording system. Cumulative concentration curves to (PHE; 30nM-100µM) and (PSE; 1-100µM; Sudafed syrup) were generated. Preparations were also electrically field stimulated (EFS, 8Hz, 30s, 300mA at 10 min intervals) and exposed to PHE (10 &100nM) and PSE (0.3 & 3µM) for 60 min.
Results: PHE (0.1-100µM) caused concentration-dependent contractions of the PSA, with a pD2 of 5.87±0.07 (n=20) and maximum response equivalent to 140.7±12.1% of the response to 60mM KCl (Fig 1 Left). But, 1-100µM PSE was practically inactive (< 5% of the response to 60mM KCl). EFS at 8Hz, 30s produced a prazosin-sensitive (not shown) contraction (24.2±2.3% of 60mM KCl, n=21) that was significantly enhanced 2-fold by the presence of 0.3 and 3µM PSE (Fig 1 Centre), but not by either 10nM or 100nM PHE (Fig 1 Right).
Figure 1: Comparison of PHE and PSE in PSA (Left) and against EFS responses (Centre and Right). The results are the mean ± sem of 6-21 observations.
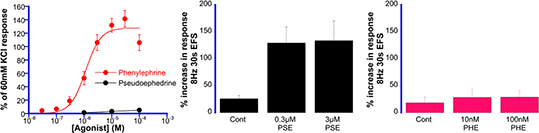
Conclusion: PHE failed to produce either a contraction of the PSA or enhance noradrenergic responses, while PSE enhanced. These findings attest to the safety of oral PHE, but comparable studies on the nasal vasculature are warranted to confirm possible efficacy as an orally-active decongestant. 1.Eccles R. (2006) Br. J. Clin. Pharmacol. 63, 10-14C. 2.Horak F et al., (2009) Ann. Allergy Asthma Immunol 102: 116-120. 3.Gelotte C and Zimmerman B (2015) Clin. Drug Investig. 35, 547-558. 4.Alijazaf et al., (2003). Br. J. Clin. Pharmacol. 56, 18-24

