Print version
Search Pub Med
| 212P London, UK Pharmacology 2016 |
Pharmacokinetics and pharmacodynamics of BIA 10-2474, a novel FAAH inhibitor, following oral administration in the Cynomolgus monkey
Background and aims
The present study reports on the relationship between exposure to BIA 10-2474 (3-(1-(cyclohexyl (methyl)carbamoyl)-1H-imidazol-4-yl)pyridine 1-oxide ) and its metabolites, fatty acid amino hydrolase (FAAH) activity in leukocytes and plasma levels of anandamide (AEA) in non-human primates.
Summary of work and outcomes
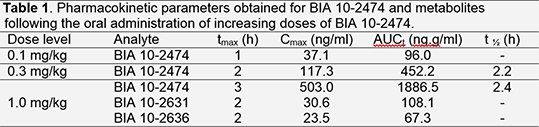
Four male Cynomolgus monkeys received the test item at three dose levels with at least a three-week washout period between administrations. Blood samples were collected until 7 days after treatment. All animal procedures were carried out in accordance with applicable European directives and Spanish laws and were subjected to ethical review by the IACUC. Plasma samples were used to determine BIA 10-2474 and its known or suspected metabolites, and to quantify AEA by LC/MS; FAAH activity was assayed in leukocytes. Pharmacokinetic parameters obtained for BIA 10-2474 and the detected metabolites, BIA 10-2631 (N-(trans-4-hydroxycyclohexyl)-N-methyl-4-pyridin-3-yl-1H-imidazole-1-carboxamide) and BIA 10-2639 (N-(trans-4-hydroxycyclohexyl)-N-methyl-4-(1-oxidopyridin-3-yl)l-1H-imidazole-1-carboxamide) after oral administration of BIA 10-2474 are in table 1. No levels above the lower limit of quantification were measured for any known metabolite after administration of BIA 10-2474 at 0.1 or 0.3 mg/kg except for one animal that showed levels of BIA 10-2631 at 1 and 2 hours after administration.
As shown in figure 1, the dose-dependent increase in BIA 10-2474 exposure (A, and the inset) was accompanied by a prolonged, but reversible, inhibition of FAAH (~50% recovery at 168 h post dose) (B) that correlated with increased plasma AEA in comparison with the pre-dose levels (C).
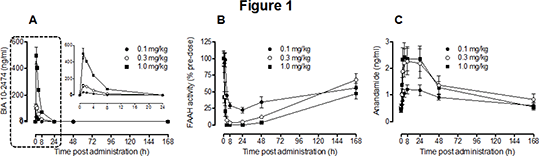
Conclusion: These data demonstrate that BIA 10-2474 is a long lasting inhibitor of FAAH in the Cynomolgus monkey and that peak effects on FAAH and AEA are attained at doses giving a Cmax of 117.3 ng/ml.

