Print version
Search Pub Med
| 216P London, UK Pharmacology 2016 |
Metabolism of BIA 10-2474, a novel FAAH inhibitor, and its interaction with Cytochrome P450 enzymes
Introduction: BIA 10-2474 (3-(1-(cyclohexyl(methyl)carbamoyl)-1H-imidazol-4-yl)pyridine 1-oxide) is a novel fatty acid amino hydrolase (FAAH) inhibitor. The present study reports on its metabolism to assist its clinical development.
Methods: The metabolite profile in plasma was assessed following oral administration with a high dose of BIA 10-2474 collected from studies involving rats, dogs and non-human primates. Analysis involved LC-MS/MS following sample precipitation. Identification of metabolites was made by comparison of retention times, mass spectra and product ion spectra with reference items. The ability of BIA 10-2474 to inhibit cytochrome P450 (CYP) enzymes in human liver microsomes was also investigated. Metabolism of selective CYP substrates by human liver microsomes was measured in the presence of six concentrations of BIA 10-2474 (0.1-30 µg/mL). Probe substrate incubates were analysed by HPLC with online radiodetection or LC-MS/MS. For assessment of time-dependent inhibition, a pre-incubation period of 30 min of BIA 10-2474 in the presence and absence of co-factor was included prior to co-incubation with CYP probe substrates. Induction of hepatic drug metabolising enzymes was assessed by pre-incubating human hepatocytes with BIA 102474 (3- 30 µg/mL) for 72 h.
Results: The major metabolites (Figure 1) were formed by hydroxylation of the cyclohexyl ring of the parent in either the meta (BIA 10-3827) or the para position (BIA 10-2639). Other identified metabolites were formed by demethylation (BIA 10-2583) or reduction of the pyridine 1-oxide (BIA 10-2445). Secondary metabolites formed by hydroxylation of the reduced form were also identified, as were glucuronide conjugates. Co-incubation of BIA 10-2474 with probe substrates revealed minor inhibition in CYP2D6 and CYP3A4 (85.1% and 86.6% of control activity, respectively) but no effects were observed following pre-incubation. No effects on CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 or CYP2E1 were observed in this study. The induction index was below the 40% threshold for CYP2B and CYP3A. At the concentrations examined in this study, BIA 102474 did not produce a cytotoxic effect.
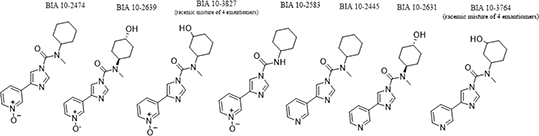
Figure 1. Chemical structures of BIA 10-2474 and synthesized metabolites
Conclusion: BIA 10-2474 bio-transformation was broadly similar between species and occurred mainly via hydroxylation of the cyclohexyl ring, although glucuronidation and nitro-reduction of the pyridine 1-oxide also take place. BIA 102474 was not considered a time-dependent inhibitor of the CYPs analysed and produced only minor inhibition of CYP2D6 and CYP3A4 but not other CYPs studied in vitro. In addition BIA 10-2474 was not considered to be an inducer of CYP2B and CYP3A.

