Print version
Search Pub Med
| 223P London, UK Pharmacology 2016 |
An updated systematic review and meta-analysis of the effect of medications with anticholinergic activity on the risk of dementia, mild cognitive impairment and cognitive decline
Introduction Dementia affects 46.8 million people worldwide and costs $818 billon in healthcare annually (1). No treatment exists to reverse dementia and so dementia prevention is a priority. Medications with anticholinergic properties are commonly used and may contribute to cognitive decline and dementia, but research to date has been conflicting (2). Here we review the evidence for an effect of anticholinergic use on dementia risk, mild cognitive impairment (MCI) and cognitive decline.
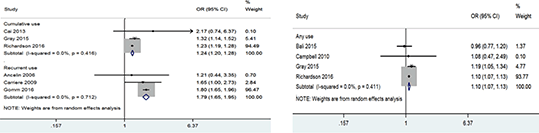
Method This review updates a previous review conducted in 2013 (2). A new search was undertaken for studies published between 2013 and 2016, with ≥12 weeks follow-up between anticholinergic medication exposure and outcomes of dementia, MCI or cognitive decline in participants aged ≥50 years. We excluded studies defining exposure via serum sample analysis alone. All studies included in our earlier review were also screened against these selection criteria. Adjusted odds ratios (OR) were pooled across similar definitions of exposure using random effects meta-analysis separately for outcomes of dementia and MCI. Unadjusted ORs were used when adjusted were unavailable. Study risk of bias was assessed using ROBINS-I (3).Figure 1. Meta-analysis of odds ratios for dementia by definite anticholinergic medication use, stratified by extent and frequency of exposure
Results 15 cohort studies and one case-control study met our criteria, although 15 had a serious or critical risk of bias. Eight studies examined incident dementia. ‘Cumulative’ (90 or more daily doses of exposure), ‘recurrent’ (used at least at two time points at least for one-year apart) or any use of drugs with ‘definite’ anticholinergic properties was associated with incident dementia (OR 1.10 -0, I2=0%; OR 1.24-0 I2=0%; and OR 1.79 -0 I2=0%) (figure 1). Any use was associated with MCI in two pooled studies (OR 1.36 [95% CI 1.03-1.80], I2=0%). It was not possible to quantitatively pool the nine studies examining cognitive decline. The study search is ongoing.
Conclusions Studies suggest that cumulative and recurrent use of medications with definite anticholinergic properties is associated with an increased risk of dementia and MCI, but studies are at risk of considerable bias. Prescribers should be cautious in prescribing anticholinergics in patients at risk of dementia and MCI.
Funding This research was supported by funding from Alzheimer’s Society (AS-PG-2013-017).
References: 1. Alzheimer’s Disease International. World Alzheimer Report 2015. London: ADI2. Fox et al (2014). Age and Ageing 43:604-615. 3. Sterne et al (2016). BMJ 355:i4919