Print version
Search Pub Med
| 228P London, UK Pharmacology 2016 |
Use of cAMP-Epac reporter mice to explore prostacyclin signaling in cardiac myocytes: a proof of concept study
Introduction: Prostacyclin is a ubiquitous cardioprotective hormone produced predominately by endothelial cells lining the surface of blood vessels and the heart. Prostacyclin protects the cardiovascular system by activating cell surface IP receptors linked to adenylate cyclase and PPARβ which is a cytosolic nuclear receptor. Adenylate cyclase is a membrane bound enzyme that converts ATP to the second messenger molecule cAMP which then engages downstream effectors such as protein kinase A or exchange protein activated by cAMP (Epac). The prostacyclin pathway has been exploited clinically with the development of drugs for the treatment of pulmonary arterial hypertension. However, the clinical utility of prostacyclin pathways is limited by an incomplete understanding of (i) the relative contribution of IP and PPARβ receptors in particular target cells, (ii) how this might be affected by disease and (iii) of how adenylate cyclase and cAMP traffics within specific cells. Recently biosensors have been developed to allow for the cellular visualisation and quantification of cAMP related signalling pathways. However, cAMP biosensing approaches have been generally limited to the study of β-adrenergic agonists and, to our knowledge, not previously been applied to prostacyclin pathways.
Methods: Here we have used Epac1-camps mice (1), which constitutively express a YFP-Epac1-CFP construct in their cells, to study cAMP responses in adult isolated cardiac myocytes. Activation of the biosensor was measured using fluorescence microscopy as YFP/CPP FRET ratio. Cells were stimulated with (i) the prostacyclin drug treprostinil, which activates both the IP and PPARβ receptors, (ii) MRE269 that selectively activates IP receptors, and GW0742, which selectively activates PPARβ receptors (each at 0.01-10 μM) followed by the combination IBMX (100μM) and forskolin (10 μM) to maximally activate adenylate cyclase.
Results: All 3 drugs tested caused concentration dependent increases in cAMP activity in cardiomyocytes as measured by FRET imaging of cells from Epac1-camps mice (Fig. 1).
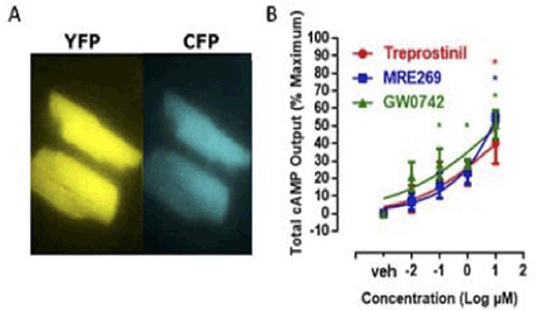
Figure 1: FRET analysis of cAMP responses in cardiomyocytes from Epac1-camps mice. (A) Images of resting cardiac myocytes from Epac1-camps mice under CFP and YFP excitation conditions. (B) cAMP levels are pharmacological activation as % maximum. Data the mean ± S.E.M. for n=3-4.
Conclusions: cAMP responses induced in cardiac myocytes by prostacyclin pathways can imaged and quantified in living cells using the Epac biosensor and FRET. This technology can now be applied to investigate subcellular compartmentalisation and trafficking of cAMP within cardiac myocytes in response to prostacyclin which will provide new therapeutic avenues for the treatment of cardiovascular disease.

