| 235P London, UK Pharmacology 2016 |
Development of a PKPD model of dual-therapy for gout to assess the impact of medication adherence on treatment success
Introduction
Effective treatment of gout requires a sustained reduction in serum uric acid (UA) to below saturation concentrations (approximately 6 mg/dL). Despite a range of urate lowering therapies (ULTs) being available the proportion of patients treated successfully has remained low (typically 50-60%).1 The purpose of this study was to aggregate available data on two ULTs, febuxostat and lesinurad, and to conduct simulation modelling in order to obtain insights into how poor adherence may influence the effectiveness and renal safety of dual-therapy for gout.
Method
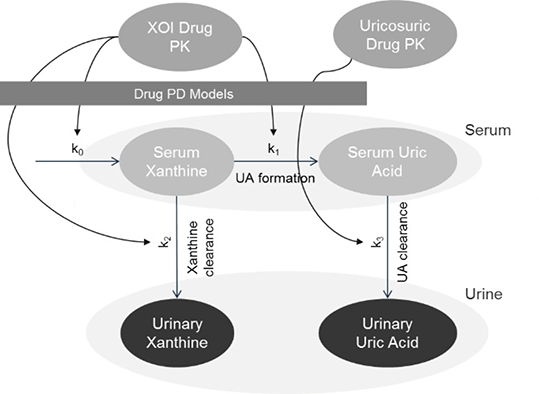
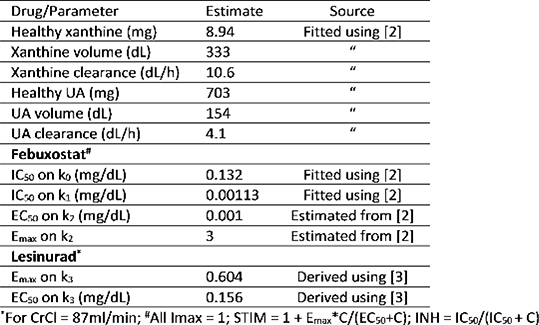
2-compartment PK models were used based on published evidence. A 4-compartment pharmacodynamic model with inhibitory and stimulatory indirect effect models was developed to capture the underlying physiology and pharmacology of the ULTs on the metabolic pathway for UA formation and elimination. Data were taken from previous PKPD studies.2,3 Additional parameters (Table 1) were estimated using nonlinear mixed effects modelling in NONMEM 7. The PKPD model was used to simulate mono and dual-ULT in gout patients with either under-excretion (lowered clearance) or overproduction of uric acid, with varying adherence modelled as randomly missed doses.
Results
For all ULTs the proportion of responders (UA < 5mg/dL) falls rapidly with worsening adherence, e.g. from 90% with perfect adherence to 44% at 50% adherence for 80mg febuxostat and 200mg lesinurad dual-therapy in under-excreters. Rates of hyperuricosuria (urinary UA > 800mg/day) were also increased with poor adherence to lesinurad and febuxostat dual-ULT. For 10% adherence to 80mg febuxostat and 200mg lesinurad dual-therapy, 1.9% of under-excreters had hyperuricosuria on at least one day. By comparison, patients whose hyperuricemia resulted from overproduction of UA up to 89.9% had hyperuricosuria in the same scenario.
Conclusion
Simulations of gout patients receiving dual-therapy shows a rapid decline in effectiveness in terms of the proportion of patients achieving target serum UA levels with worsening adherence. Under poor adherence with dual-therapy the proportion of patients predicted to have hyperuricosuria also increased, which may explain the high rate of renal adverse events in clinical trials.3 This may add to the safety concerns for lesinurad given that adherence to ULTs is often very poor4 and that hyperuricosuria is associated with kidney disease5.
References
1. Bernal et al. (2016). Ther Adv Chronic Dis. 7(2): 135-44
2. FDA. Febuxostat Clin Pharm Review. 2008.
3. FDA. Lesinurad Clin Pharm Review. 2014.
4. Halpern R et al. (2009). Curr Med Res Opin. 25(7): 1711-1719
5. Li et al. (2014) BMC Nephrol. 15: 122