Print version
Search Pub Med
| 019P London, UK 8th European Workshop on Cannabinoid Research |
The effects of pharmacological blockade of PPARs on formalin-evoked nociceptive behaviour, fear-conditioned analgesia and conditioned fear in the presence of nociceptive tone in rats
Introduction Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors1. There is evidence for their involvement in pain2 and cognition3, however, their role in pain-fear interactions is unknown. The basolateral amygdala (BLA) plays a key role in pain, conditioned fear and fear-conditioned analgesia (FCA). This study investigated the effects of systemic or intra-BLA administration of PPARα, PPARβ/δ and PPARγ antagonists on formalin-evoked nociceptive behaviour, FCA, and conditioned fear in the presence of nociceptive tone in rats.
Methods Male Sprague-Dawley (SD) rats received footshock in a conditioning arena. 23.5 hours later, rats received intraplantar injection of formalin (2.5%, 50 μl) into the right hind paw and intraperitoneal administration of vehicle (1:1:8, ethanol: cremophor: saline 0.9%), PPARα (GW6471; 2mg/kg), PPARβ/δ (GSK0660; 1mg/kg) or PPARγ (GW9662; 2mg/kg) antagonists before re-exposure to the conditioning arena. Nociceptive and fear-related behaviours were assessed and tissue levels of neurotransmitters/endocannabinoids measured in the BLA and central amygdala (CeA) by LC-MS/MS. In a second experiment, male SD rats underwent the same protocol; after formalin administration, antagonists at PPARs (10 μg/0.5 μl), or vehicle (DMSO 100%/0.5 μl), were microinjected bilaterally into the BLA and their effects on pain- and fear-related behaviours were assessed. The experimental procedures were approved by the animal Care and Research Ethics Committee, National University of Ireland Galway, and the work was carried out under license from Health Products Regulatory Authority in the Republic of Ireland and in accordance with EU Directive 2010/63.
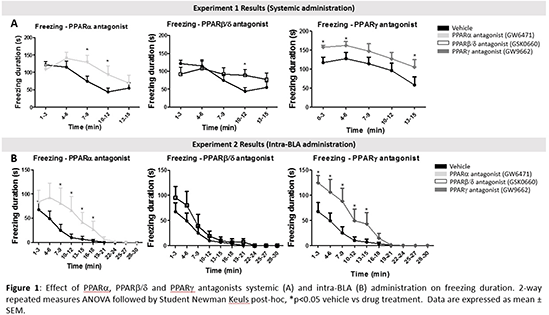
Results Systemic administration of PPARs (n=9/group) antagonists potentiated context-induced freezing without altering nociceptive behaviour when compared to vehicle-treated counterparts (n=9) (Figure 1). PPARα and PPARβ/δ antagonists increased GABA levels (2-way ANOVA followed by Fisher LSD post-hoc; VehiclevsGW6471, p=0.012; VehiclevsGSK0660, p=0.007) in the contralateral BLA and PEA levels (Vehicle vs GW6471, p=0.021; Vehicle vs GSK0660 p=0.016) in the ipsilateral CeA. Intra-BLA administration of GW6471 (n=6) or GW9962 (n=7) potentiated context-induced freezing (Figure 1).
Conclusions PPARs, and particularly PPARα and PPARγ expressed in the BLA, may play a role in within-trial extinction of conditioned fear in the presence of nociceptive tone. Further work is required to determine the extent to which the neurochemical alterations observed are causally implicated in PPAR-mediated regulation of fear-related behaviour.
Acknowledgements This study was supported by funding from CNPq - Brazil (207530/2014-9).
References
1Blanquart C. et al. (2003). J. Steroid Biochem. Mol. Biol. 85: 267-73.
2LoVerme J et al. (2006) J Pharmacol Exp Ther 319: 1051-61
3Panlilio L. et al. (2013) Pharmacol Ther 84-102