| 006P London, UK Pharmacology 2017 |
Determination of Association (kon) and Dissociation (koff) Rates of inhibitors of the PI3Kδ isoform using the LanthaScreen® technology
Introduction: There is an increasing evidence of potential advantages on duration of pharmacodynamics and target selectivity for kinase inhibitors with long residence time. Obtaining kinetic parameters at early stage of drug discovery may allow improved predictions of in vivo drug efficacy (1). PI3K kinases inhibitors have been studied in a variety of inflammatory processes and hematologic malignancies (2).
Methods: In order to obtain kon and koff values of inhibitors at human PI3Kδ kinase isoform, a simple non-radioactive method was setup and the binding kinetics of reference inhibitors CAL-101 (3), IPI-145 (4) and SW13 (5) were determined by using Motulsky and Mahan method (6). The LanthaScreen® (ThermoFisher) binding of Tracer-314 to PI3Kδ-His enzyme coupled to Eu-anti-His antibody produced an increase of Fluorescence Resonance Energy Transfer (FRET), which was measured with the Envision plate-reader. PI3Kδ-Eu kinase and tracer titration curves were measured after 1h incubation at 23°C. The tracer saturation curve gave an average KD value of 8.1nM (pKD=8.09±0.04, average±StDev, n=3). Increasing tracer concentrations were associated to a fixed amount of PI3Kδ-Eu. Kon was calculated as 5.7E7M-1min-1 and koff 0.47min-1 and the KD calculated as from koff/kon was 8.1nM. Tracer koff average value from dissociation experiments was 0.41min-1 (n=3), corresponding to a 2min dissociation half-life (t1/2off). Tracer kon was also calculated from dissociation and saturation experiments, (koff/KD, 5.1E7M-1).
Results: The kinetics binding properties of the three PI3Kδ reference inhibitors were analyzed. Tracer was associated to PI3Kδ-Eu with increasing inhibitor concentrations. Data were analyzed using GraphPad v5 software. The t1/2offfor CAL-101, IPI-145 and SW13 was 2, 6 and 12min, respectively. The inhibitor affinities (KD) were calculated as koff/kon ratio and were compared with the potency values obtained in a functional ADP-Glo enzymatic assay (Promega). A good correspondence between inhibitor affinities and potencies was found (Table 1).
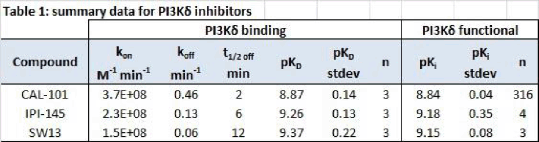
Conclusions: The LanthaScreen® technology allowed the determination of kinetics properties of the three PI3Kδ inhibitors. CAL-101 was the faster dissociating PI3Kδ kinase inhibitor whereas SW13 the slowest. These results may have an implication on their pharmacodynamics.
References:
1. Copeland R (2006). Nature Rev Drug Discov. 5: 730-739.
2. Wang X et al. (2015). Acta Pharmacol Sin. 36 (10): 1170-1176.
3. Somoza JR et al. (2015). J Biol Chem. 290 (13): 8439-8446.
4. Winkler DG et al. (2013). Chem Biol. 20: 1364-1374.
5. Williams O et al. (2010). Chem Biol. 17 (2): 123-144.
6. Motulsky HJ and Mahan LC (1984). Mol Pharmacol. 25: 1-9.

