| 007P London, UK Pharmacology 2017 |
Physiological consequences of RAMP-mediated agonist bias at the calcitonin receptor-like receptor
Introduction: The calcitonin receptor-like receptor (CLR) is a G protein-coupled receptor (GPCR) formed of a heterodimer between the receptor, and a receptor activity-protein (RAMP)1. The endogenous peptide ligands for this receptor are calcitonin gene-related peptide (CGRP), adrenomedullin (AM) and adrenomedullin 2 (AM2), and have cardiovascular protective actions2. So far signalling bias at the CLR has been documented in heterologous cell lines3. Here we extend these studies to the physiological system of Human umbilical vein endothelial cells (HUVECs).
Method: HUVECs (pooled donors) were cultured (passage 1-6). RT-PCR was performed to determine the CLR and accessory protein composition of the cells. Assays for cAMP accumulation, intracellular Ca2+ release and ERK phosphorylation (pERK1/2) were perform as described3.
Figure 2. To enable comparison of the extent of signalling bias, a ‘web of bias’ was calculated3.
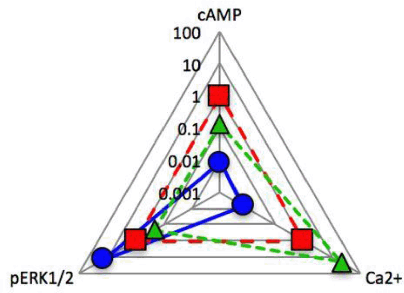
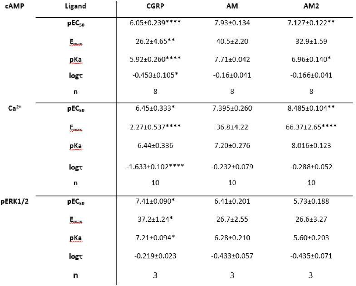
Results: The RT-PCR data indicated that HUVECs express CLR and RAMP2 hence generating an adrenomedullin (AM) receptor. We were unable to detect the presence of mRNA for either RAMP1 or RAMP3 in our cells. Each agonist was shown to evoke signals via the 3 main signalling pathways explored (Figure 1). Importantly the 3 agonists demonstrated clear pathway bias, as determined through the application of the operational model of agonism (Figure 2). CGRP displayed clear bias towards ERK1/2 phosphorylation, while AM was biased towards cAMP production, and intriguingly AM2 appears to display a previously unreported bias towards Ca2+ release.
Figure 1. Agonism data for the ligands at the AM receptor. Statistical significance to AM calculated using a one-way ANOVA with Bonferroni’s post-test (*,p<0.05,**,p<0.01 and ***p<0.001).
Conclusion: These results suggest a potential physiological role for signalling bias. Our next steps will explore how these ligands influence processes such as NO release and endothelial cell permeability.
References:
1. MCLATCHIE, L. M. et al. (1998) Nature, v. 393 p. 333-9;
2. RUSSELL, F. A. et al. (2014) Physiol Rev, v. 94, n. 4, p. 1099-142.
3. WESTON, C. et al. (2016) J Biol Chem, v. 291, n. 42, p. 21925-21944.
This work was funded by AstraZeneca, a Welcome Trust Summer Scholarship and the BBSRC (- BB/M00015X/2) and an MRC Doctoral Training Partnership (MR/J003964/1).

