Print version
Search Pub Med
| 038P London, UK Pharmacology 2017 |
The emerging therapeutic role of KARs and Netos against neuropsychiatric disorders
Introduction: Kainate receptors (KARs) are ionotropic glutamate receptors involved in presynaptic and postsynaptic neurotransmission mechanisms. They form functional ion channels by tetrameric combinations of five different subunits (GRIK1-GRIK5, GluK1-GluK5) modulated by auxiliary proteins Neto1 and Neto2. This study will investigate how rare disruptive genetic mutations will affect KAR ionic function (1),(2).
Methods: We used bioinformatic analysis of next generation sequencing data to interpret how rare coding variants may contribute to neuropsychiatric diseases in patient populations. We employed voltage-clamp electrophysiological recording of Xenopus oocytes expressing cloned human KARs and treatment with agonist compounds. We also assessed the effect of protein damaging mutations on the electrophysiological and pharmacological properties of human KARs.
Results: We report the identification of a number of rare predicted damaging coding mutations, GluK4(Y555N) and GluK4(L825W), found exclusively in diseased individuals and which may affect the expression and the physiological properties of KARs. We also report GluK2 and GluK2/GluK4 subunit sensitivities to agonist compounds and their current decay kinetics. We demonstrate that the two functional missense mutations reduce the agonist sensitivity (extra sum of squares F-test: p<0.05) (figure 1) and alter the decay kinetics of heteromeric GluK2/GluK4 receptors (unpaired t-test: p<0.05).
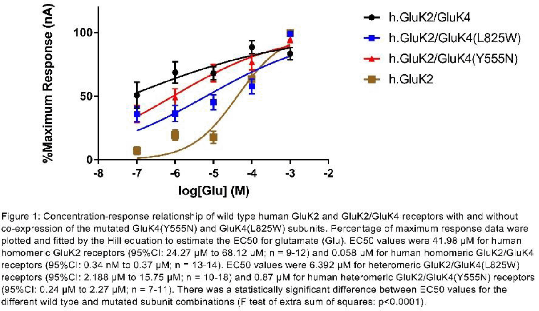
Conclusion: Our current findings indicate that functional genetic mutations within KARs alter their pharmacological and electrophysiological properties. Future work will involve investigating the effect of functional genetic mutations on the properties of human KARs when Neto auxiliary proteins are being co-expressed. This research will contribute to a better understanding of the link between genetic risk, biological processes and potential therapeutic avenues for brain diseases.
References:
1. FRANK, R. A., et al., (2011). "Clustered coding variants in the glutamate receptor complexes of individuals with schizophrenia and bipolar disorder." PLoS One 6(4): e19011.
2. KNIGHT, H. M. et al., (2012). "GRIK4/KA1 protein expression in human brain and correlation with bipolar disorder risk variant status". Am J Med Genet B Neuropsychiatr Genet, 159: 1: 21-9, 2012.

