Print version
Search Pub Med
| 040P London, UK Pharmacology 2017 |
Identification of small molecules binding to the cAMP effector protein Popeye domain containing 1 (POPDC1)
Introduction: The Popeye domain containing genes encode a novel class of membrane-bound cAMP effector proteins (1). Homology modelling of the Popeye domain domain revealed structural similarity to other cAMP-binding domains, while the cyclic nucleotide-binding cassette is highly divergent (2). POPDC proteins are involved in the control of membrane trafficking of ion channels in a cAMP-dependent manner. Loss-of function mutations in mouse and zebrafish models and patients, carrying a mutation in POPDC1 display cardiac arrhythmia and muscular dystrophy (2-4). In order to identify compounds, which selectively target POPDC proteins, we developed a small molecule screen using recombinant POPDC1 protein.
Method: The Popeye domain of POPDC1 was expressed as a recombinant GB1 fusion protein. The cAMP-binding domains of EPAC1 and EPAC2 served as controls. A set of pharmacophores based on cAMP binding to POPDC1 were generated and used to screen Domainex NICE database of over one million lead-like commercially available compounds. 500 compounds were prioritised for testing using three different assays: (a) ELISA measuring binding of a biotin containing analogue of cAMP (2-Biotin-AHA-cAMP) and detected by HRP-conjugated streptavidin, (b) Differential Fluorimetry Scanning (DSF) to confirm binding and (c) tryptophan scanning to assess the KD (n=3 in each assay).
Results: In order to identify compounds, which selectively bind to the Popeye domain of POPDC1, we set out to develop a suitable binding assay. Attempts to develop an assay using 8-NBD-cAMP failed, as binding to POPDC1 did not cause a change in fluorescence. We therefore made use of 2-Biotin-AHA-cAMP as a ligand in an ELISA-based screening assay. In order to reduce the number of molecules that needed to be tested, we employed a pharmacophore virtual screening approach. A total of 500 compounds prioritised were used in the ELISA, Trp scanning and melting temperature analysis. Seven compounds bound competitively to the Popeye domain and several of these demonstrated selectivity (Fig. 1 and Table 1).
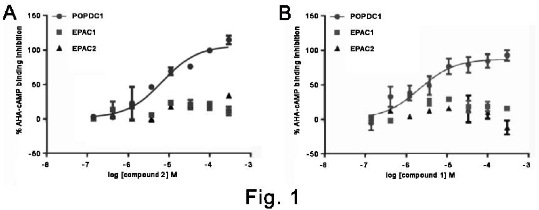
Table 1: POPDC1 binding affinities of identified compounds
| Compound # | IC50 [μM] | Trp Scanning [μM] | ΔTm [°C] |
| 1 | 2.11 | 49.0 | -0.66 |
| 2 | 6.83 | 134 | -1-08 |
| 3 | 0.42 | 100 | -0.94 |
| 4 | 2.29 | 206 | -1.66 |
| 5 | 5.68 | 355 | +2.13 |
| 6 | 2.23 | 124 | -0.62 |
| 7 | 1.12 | 27.8 | -1.03 |
Conclusions: Seven small molecules were identified, which bind to the Popeye domain of POPDC1. The next step will be to further characterise their biological properties and find out whether they are suitable to agonise or antagonise the functions of members of the POPDC protein family.
References
(1) Schindler, RF and Brand T (2016). Progr Biophys Mol Biol 120: 28-36.
(2) Froese A et al. (2012). J Clin Invest 122: 1119-1130.
(3) Kirchmaier B et al. (2012). Dev Biol 363: 438-450.
(4) Schindler RF et al. (2016). J Clin Invest 126: 239-253.

