Print version
Search Pub Med
| 044P London, UK Pharmacology 2017 |
Characterising glucose-dependent insulinotropic polypeptide receptor signalling
Introduction: The incretins, glucose-dependent insulinotropic polypeptide (GIP (1-42)) and glucagon-like peptide 1 (GLP-1 (7-36)NH2) stimulate insulin secretion from pancreatic beta-cells. However, they have contrasting regulatory actions on glucagon secretion from pancreatic alpha-cells with GIP (1-42) stimulating glucagon secretion and GLP-1 inhibiting its release (1). Incretins are thus potential targets for treating type 2 diabetes mellitus. Whilst GLP-1R signalling has been comprehensively studied and an interaction with receptor activity-modifying proteins (RAMPs) proven not to exist, GIPR signalling is relatively underexplored and it is not known whether RAMPs can interact with the GIPR.
Method: Flow cytometry and enzyme-linked immunosorbent assays, with APC and HRP-conjugated anti-FLAG antibodies, respectively, were used to detect levels of FLAG-RAMP surface expression when coexpressed with GIPR. Plasma membrane colocalisation of GIPR-mCherry and RAMP-GFP was determined using confocal microscopy. Mammalian HTRF-based second messenger assays for cAMP accumulation, ERK1/2 phosphorylation (8 and 5 minute stimulation, respectively) and intracellular calcium ((Ca2+)i) mobilisation (Fluo4/AM) were utilised to measure the response of HEK-293 cells transiently expressing GIPR to GIP (1-42) in the presence and absence of RAMP1, 2 and 3. The operational model of pharmacological agonism was used to determine the efficacy of GIP (1-42) at each GIPR-RAMP complex (2). Data are given as mean±SEM (n individual repeats) and analysis was performed using one way ANOVA and Dunnett's post-test.
Results: Coexpression of GIPR with each RAMP led to significant increases in APC intensity and HRP activity relative to RAMPs coexpressed with empty vector (Table 1). Confocal microscopy revealed membrane colocalisation between GIPR-mCherry and each RAMP-GFP. Coexpression of GIPR with RAMP1 significantly reduced the efficacy of GIP (1-42) to stimulate (Ca2+)i mobilisation (p<0.01, n=4), whilst coexpression with RAMP2 attenuated the efficacy for both (Ca2+)i mobilisation (p<0.0001, n=4) and ERK1/2 phosphorylation (p<0.0001, n=4). Interestingly, coexpression with RAMP3 had no effect upon (Ca2+)i mobilisation, but reduced the efficacy of GIP (1-42) to stimulate cAMP accumulation (p<0.05, n=9, Figure 1).
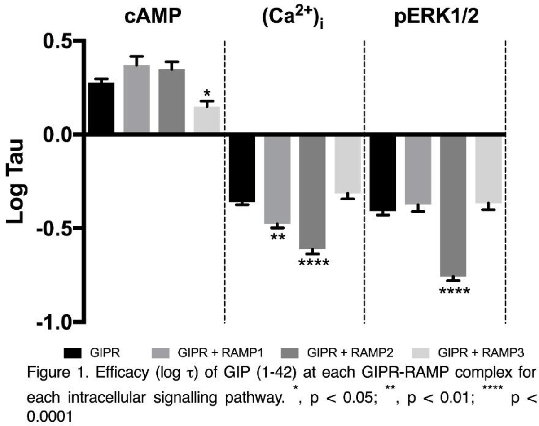
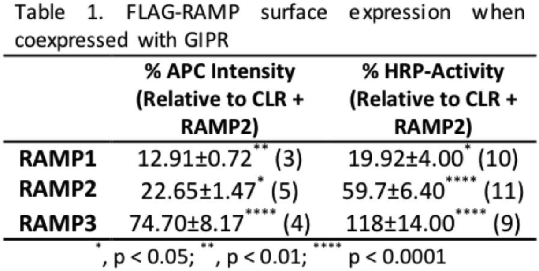
Conclusions: These data provide evidence for GIPR as a novel RAMP interacting GPCR. Interaction with each RAMP resulted in RAMP-specific modulation of GIPR signalling. The differential modulation of GIPR activity by RAMPs could indicate different roles for GIPR in tissues expressing different RAMPs opening up the possibility of developing anti-diabetic drugs that target specific GIPR-RAMP combinations.
References:
(1). Seino Y et al. (2010). J Diabetes Investig 1: 8-23.
(2). Black JW and Leff P (1983). Proc R Soc L. B Biol Sci 220: 141-162.

