Print version
Search Pub Med
| 050P London, UK Pharmacology 2017 |
Network meta-analysis of cardiovascular outcomes in randomised controlled trials of new classes of antidiabetic drugs
Introduction: The prevalence of cardiovascular disease is high in patients with type 2 diabetes mellitus (T2DM). New antidiabetic drugs are required to demonstrate cardiovascular safety in outcome trials. However, they are rarely compared with each other in such trials. Therefore, we performed a network meta-analysis to compare the cardiovascular outcomes of the new classes of antidiabetic drugs.
Method: We searched for randomised controlled trials involving glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose co-transporter 2 (SGLT-2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors in T2DM patients with cardiovascular outcomes, namely, major adverse cardiovascular events (MACE) and mortality, as endpoints. Both frequentist approach and Bayesian framework were used for analysis in R.
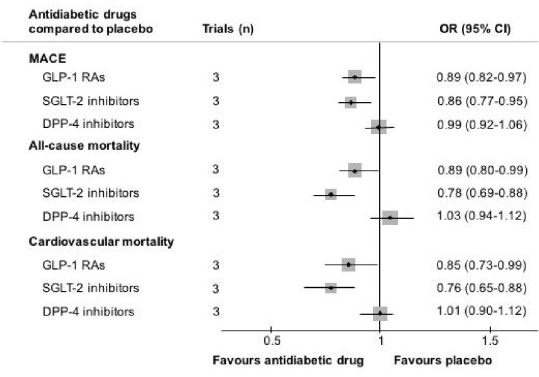
Results: Nine randomised controlled trials with altogether 72262 T2DM patients were included in our network meta-analysis -8. SGLT-2 inhibitors and GLP-1 RAs reduced MACE (OR 0.86, 95%CI 0.77-0.95 and 0.89, 0.82-0.97, respectively), all-cause mortality (0.78, 0.69-0.88 and 0.89, 0.80-0.99, respectively), and cardiovascular mortality (0.76, 0.65-0.88 and 0.85, 0.73-0.99, respectively) when compared to placebo. Moreover, SGLT-2 inhibitors reduced MACE (0.87, 0.77-0.98), and cardiovascular mortality (0.75, 0.62-0.90) when compared to DPP-4 inhibitors. In contrast, DPP-4 inhibitors were not significantly different from placebo in cardiovascular outcomes but were associated with higher all-cause mortality when compared to SGLT-2 inhibitors (1.31, 1.13-1.53) and GLP-1 RAs (1.16,1.01-1.33).
Conclusions: SGLT-2 inhibitors and GLP-1 RAs reduce MACE and mortality compared to placebo. DPP-4 inhibitors are safe in that they do not worsen cardiovascular outcome, but are inferior to SGLT-2 in terms of MACE and cardiovascular mortality. This network meta-analysis shows clearly that SGLT-2 inhibitors and GLP-1 RAs should be the preferred treatment.
References:
1. Marso SP et al. (2016). N Engl J Med 375: 311-322.
2. Marso SP et al. (2016). N Engl J Med 375: 1834-1844.
3. Pfeffer MA et al. (2015). N Engl J Med 373(23): 2247-57.
4. Zinman B et al. (2015). N Engl J Med373(22): 2117-28.
5. Neal B et al. (2017). N Engl J Med 377(7): 644-657.
6. Scirica BM et al. (2013). N Engl J Med 369(14): 1317-26.
7. Green JB et al. (2015). N Engl J Med 373(3): 232-42.
8. White WB et al. (2013). N Engl J Med369(14): 1327-35.
Trials included in the network meta-analysis
| Study | Drug | Drug class | Reference |
| LEADER | Liraglutide | GLP-1 RA | Marso SP et al. (1) |
| SUSTAIN-6 | Semaglutide | GLP-1 RA | Marso SP et al. (2) |
| ELIXA | Lixisenatide | GLP-1 RA | Pfeffer MA et al. (3) |
| EMPA-REG OUTCOME | Empagliflozin | SGLT-2 inhibitor | Zinman B et al. (4) |
| CANVAS | Canagliflozin | SGLT-2 inhibitor | Neal B et al. (5) |
| CANVAS-R | Canagliflozin | SGLT-2 inhibitor | Neal B et al. (5) |
| SAVOR-TIMI 53 | Saxagliptin | DPP-4 inhibitor | Scirica BM et al. (6) |
| TECOS | Sitagliptin | DPP-4 inhibitor | Green JB et al. (7) |
| EXAMINE | Alogliptin | DPP-4 inhibitor | White WB et al. (8) |

