Print version
Search Pub Med
| 060P London, UK Pharmacology 2017 |
Effect of C terminus truncations on light induced signalling and internalisation of optogenetic rhodopsin-α1A adrenoceptor chimeras
Introduction: Chimeric opsin-G protein coupled receptors (OptoXR) are a powerful method for optogenetically dissecting receptor signalling. They include the α1A adrenoceptor-OptoXR, coupled to Gq which increases intracellular Ca2+ upon light stimulation (1). The wild type α1A adrenoceptor internalisation mechanism is not clear, however the α1A adrenoreceptor binds to β-arrestin and contains several intracellular phosphorylation sites (2). In order to generate OptoXRs with distinct spatiotemporal signalling profiles, we designed C tail two truncated receptors targeting the C tail phosphorylation sites of the α1A-OptoXR, and compared their Ca2+ response profiles and internalisation in response to light stimulation.
Methods: SNAP-tagged-α1A-OptoXR cDNAs in pcDNA3.1 were truncated at Ser371 or Thr471 by PCR based mutagenesis, and stably expressed in HEK293T cells. Cells were kept in the dark prior to imaging calcium responses and receptor internalisation on a LSM-710 Confocal microscope (Zeiss), using 488 nm laser excitation to also stimulate OptoXR activation. Calcium mobilisation was assessed after loading with 10 μM Fura Red-AM (20 min, 1 fps), while receptor internalisation (30 min, 0.1 fps) was monitored after first labelling receptors with 0.5 μM SNAPsurface488, as the change in plasma membrane fluorescence intensity. Where required media was supplemented with 1 μM 9-cis-retinal for 1h prior to stimulation and in solution during the experiment. One way ANOVA with Sidak's multiple comparisons was performed and data are expressed as mean±SEM.
Results: 9-cis-retinal supplementation increased the proportion of light stimulated SNAP-α1A-OptoXR cells eliciting calcium responses (45.5%±4.1 compared to 24.0%±5.5 in the absence of retinal, n = 3), the magnitude of the peak response, and the number of Ca2+ transients within the stimulation period (10.1±0.9 compared to 5.0 ± 0.7, n = 18-56 cells, 3 experiments, p < 0.01). Ser371 truncation (but not Thr471) further increased the percentage cell responders (76.7%±5.9, n = 3, p < 0.05) and the number of calcium transients (14.3±0.9, n = 3, p < 0.05).
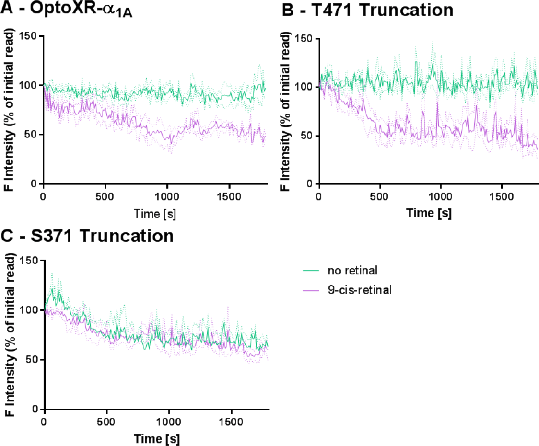
SNAP-α1A-OptoXR underwent light induced internalisation, but only in the presence of 9-cis-retinal (48.5%±2.1 initial plasma membrane intensity at 30 min). This retinal-dependent response was prevented by Ser371 truncation.
Conclusions: The SNAP- α1A-optoXR required 9-cis-retinal supplementation to produce Ca2+ transients and receptor internalisation. Cleavage at Thr471 was not sufficient to alter signalling. We conclude that through limiting receptor desensitisation, the Ser371 truncation delivers an α1A-OptoXR with sustained response kinetics.
References:
(1) Airan, R. D. et al. (2009). Nature 458: 1025-1029.
(2) Stanasila, L. et al. (2008). Molecular Pharmacology, 74: 562-573.

