Print version
Search Pub Med
| 061P London, UK Pharmacology 2017 |
Adverse drug reactions to antiretroviral therapy - a prospective observational study
Introduction: Antiretroviral therapy (ART) is a lifelong commitment for Human immunodeficiency virus infection (HIV) positive patients. The patients may develop early adverse drug reactions (ADRs) during first three months of treatment, especially when, they have advanced HIV disease with low CD4 counts. 1
Method: The present study was a prospective observational study that took place from January 2017 to July 2017 at ART Center, Government Medical College, Amritsar, Punjab, India. The primary objective of the study was to evaluate adverse drug reactions to antiretroviral therapy in HIV positive patients. The patients received ART regimens as per National AIDS Control Organization guidelines (NACO).2 Sample included both ART naïve and ART experienced HIV positive patients. The causality of reported ADRs assessed according to World Health Organization (WHO) guidelines. The study conducted after taking approval from Institutional Ethics Committee, Government Medical College, Amritsar and informed consent from the patients. Data analyzed statistically by using Chi-square test and p-value less than 0.05 considered significant.
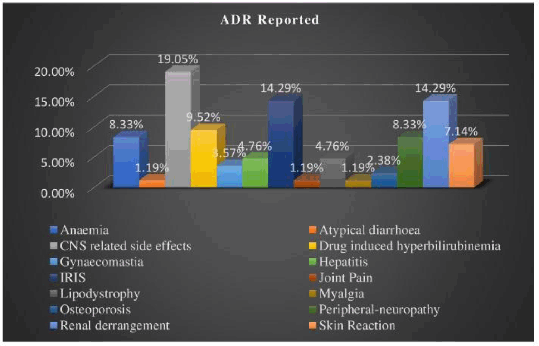
Results: Eighty-four patients reported to ART center during study period. Most widely used treatment regimen was Tenofovir+Lamivudine+Efavirenz (TLE) (59.52%). Central Nervous System (CNS) (19.05%), renal (14.29%) and IRIS (14.29%) related ADRs mostly reported. Out of 84 patients, 73.81% had mild and 14.29% severe grade of ADRs. The causality assessment revealed insignificant p-value (Chi-square value= 8.656 and p-value=0.07), with 19.05% probable and 80.95% possible ADRs. Among these patients, majority had CD4 count less than 200/μl (47.62%) at the initiation of ART and between 200-499 /μl when they reported with ADRs. Mostly the patients were found to be in WHO clinical stage I at the initiation of ART (51.19%) and when they developed ADRs (77.38%). The mean treatment duration for the development of ADRs was 2.6±2.88 (mean ± standard deviation) years. Most of the patients developed ADRs within one year of treatment (45.24%).
Conclusions: The CD4 count less than 200/μl at the initiation of ART and treatment duration less than one year proved to be predictors for ADRs. Early initiation of ART in HIV positive patients can improve immunological status and decrease in the incidence of ADRs.
References:
1. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; 2016.
2. Policy & Guidelines | National AIDS Control Organization | MOHFW | GOI. Available from: http://naco.gov.in/documents/policy-guidelines
WHO causality assessment
| WHO causality categories | TLE | ZLN | ZL+ATZr | TL+ATZr | ZLE | Total |
| Probable | 7 | 3 | 1 | 3 | 2 | 16 |
| Possible | 43 | 17 | 0 | 4 | 4 | 68 |
| Total | 50 | 20 | 1 | 7 | 6 | 84 |
| Chi-Square value= 8.656 | Degree of freedom= 4 | p-value= 0.07 | insignificant p-value |