Print version
Search Pub Med
| 063P London, UK Pharmacology 2017 |
Differences in the pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 cannabinoid receptor CB1R
Introduction: Despite almost identical structures, (-)-cannabidiol (CBD) and its derivative, (-)-cannabidiol-dimethylheptyl (CBD-DMH), exhibit distinct pharmacology in vivo that can be attributed to the type 1 and type 2 cannabinoid receptors (CB1R and CB2R).(1) The purpose of this study was to determine how the structural differences between CBD and CBD-DMH relate to their disparate pharmacology at CB1R.
Method: HEK293 cells expressing human CB1R-GFP2 ± βarrestin1-Rluc were treated with CBD or CBD-DMH ± the cannabinoid agonist CP55,940 or vehicle (1% DMSO in PBS). Inhibition of cAMP (cAMP luciferase reporter assay; Promega)(2) and βarrestin1 recruitment [bioluminescence resonance energy transfer (BRET)](3) were measured. Data were fit to the operational model of allosterism.(3) AutoDock 4.2(4) was used to simulate the binding of CBD and CBD-DMH relative to CP55,940, SR141716A (inverse agonist), and Org27569 (CB1R allosteric modulator) using the recently published crystal structures of agonist-bound (i.e. active) and antagonist-bound (i.e. inactive) human CB1R (PDB ID: 5TGZ, 5XRA).(5,6)
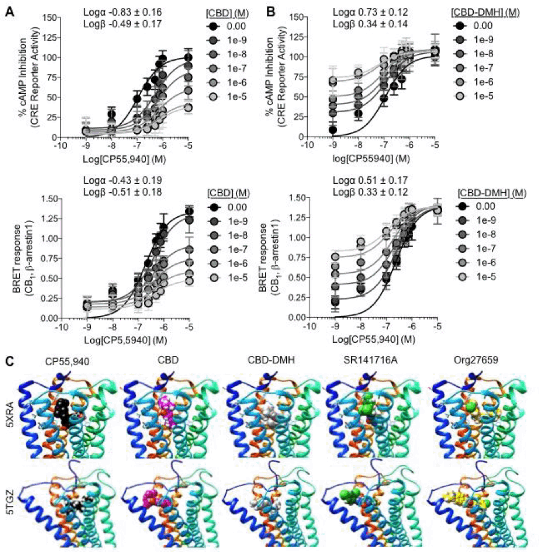
Results: CBD was a negative allosteric modulator (NAM) (n=6),(3) and CBD-DMH was a mixed agonist/positive allosteric modulator (ago-PAM) (n=6) of CP55,940 in CB1R cAMP inhibition (Fig. 1A) and βarrestin1 recruitment assays (Fig. 1B). In ligand docking simulations, CBD and Org27569 bound the outer vestibule in the inactive CB1R conformation, separate from that of CP55,940 (Fig. 1C). CBD shared a binding site with CP55,940 in the active CB1R conformation model (Fig. 1D). The observed binding site for CBD-DMH overlapped with CP55,940 and Org27569 in both active and inactive CB1R model conformations (Fig. 1C,D).
Conclusions: The pharmacological activity and modelled binding of CBD and CBD-DMH at CB1R may explain the disparate effects of these compounds observed in vivo.(1)These data suggest ligand binding to CB1R may be fluid, such that ligands transition between allosteric and orthosteric sites of action.
References:
(1) Fride E et al. (2005). Neuropharmacol 48, 1117-1129.
(2) Laprairie RB et al. (2016). ACS Chem Neurosci 7, 776-798.
(3) Laprairie RB et al. (2015). Br J Pharmacol 172, 4790-4805.
(4) Morris GM et al. (2009). J Comput Chem 16, 2785-2789.
(5) Hua T et al. (2016). Cell 167, 750-762.
(6) Hua T et al. (2017). Nature 574, 468-471.
Figure 1: CBD is a CB1R NAM. CBD-DMH is a CB1R ago-PAM. CBD (A) and CBD-DMH (B) had opposing effects on potency (Logα) and efficacy (Logβ) of CP55,940 dependent cAMP inhibition and βarrestin. (C) AutoDock simulations of CP55,940, CBD, CBD-DMH, SR141716A, and Org27569 binding to agonist-bound (5XRA) and antagonist-bound (5TGZ) models of human CB1R.