| 068P London, UK Pharmacology 2017 |
Phosphodiesterase inhibition a potential approach to glioblastoma: a preliminary study modulating cAMP levels
Introduction: Altered expression of phosphodiesterases (PDEs) and the intracellular cAMP levels have been reported to be associated with cancer (1). Increased cAMP levels inhibit cell proliferation, whereas low levels trigger severe malignancy (2). We aimed to investigate the potency of a panel of PDE inhibitors in modulating cAMP levels in cell lines associated with glioblastoma.
Methods: Semi-quantitative reverse-transcriptase PCR was used to profile the expression pattern of PDEs in U87 cells, as a model for glioblastoma (3), with comparison to HEK293S cells. Further, both cell lines were co-stimulated with forskolin (final concentration of 158 nM-the pEC30) and increasing concentrations of each PDE inhibitor (applying 100-fold range spanning the published in vitro IC50 value, (4)) for 30 minutes. Accumulation of cAMP was determined using the LANCE cAMP detection kit (PerkinElmer, Boston, MA).
Results: With the exception of PDE6 isoenzymes (absent in U87 cells) there appeared little difference in PDEs expressed between both cell lines. Significantly both cell types displayed distinct profiles for cAMP accumulation in the presence of the PDE inhibitors (Table 1). While rolipram, amrinone and trequinsin elevated cAMP levels in both cell types, EHNA and caffeine appeared only effective in the U87 cell line.
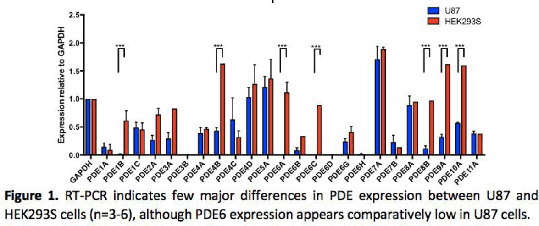
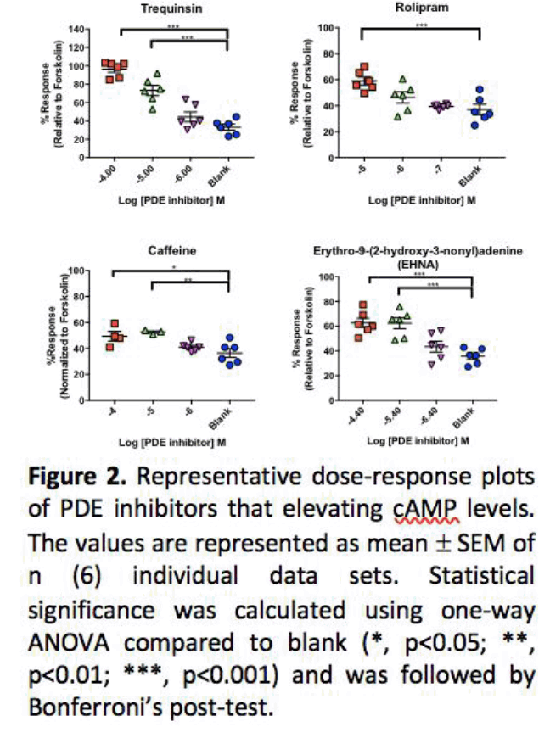
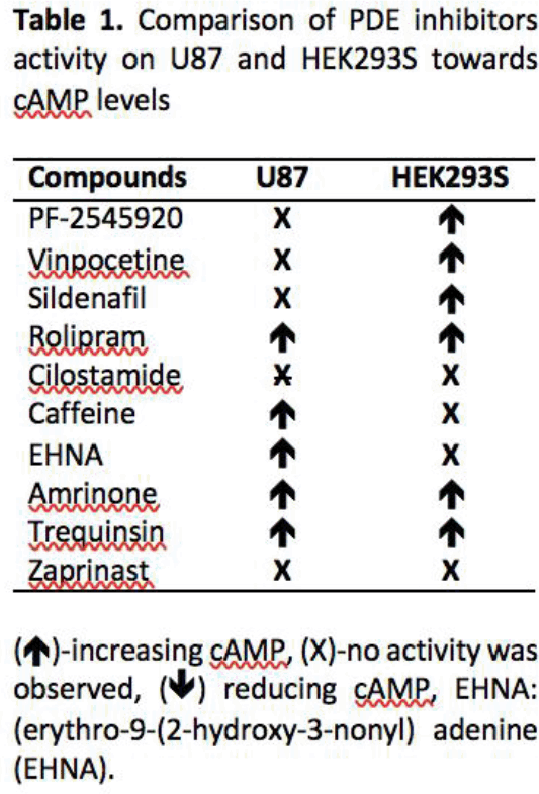
Conclusion: PDE inhibition leads to distinct patterns of cAMP accumulation in the two cell types, in part reflecting basal PDE mRNA expression but also the specificity of the PDE inhibitors. Inhibition on PDE 2, 3, 4, and 7 seem to be contributed in elevating cAMP compared to remaining PDEs. We next aim to determine if these inhibitors modulate cell proliferation in U87 cells.
References:
(1) Savai et al. (2010). Expert Opin. Investig. Drugs, 19: 117-131.
(2) Sengupta et al. (2011). Trends Pharmacol Sci, 29: 647-654
(3) Lenting et al. (2017) Acta Neuropathol. 133: 263-282.

