| 079P London, UK Pharmacology 2017 |
Translational pharmacokinetic-pharmacodynamic analysis of sedative effects in rats and humans using published data
Introduction: Drug-induced sedation can impair a person’s ability to function normally over a period of time (1), and can interfere with their daily life routine, as well as compromising drug compliance. Rodent models are often used to predict pharmacological effects in the clinic, but the translation to humans is not well-understood. We have attempted to quantify the translation of pharmacologically-induced sedation from rats to healthy volunteers using pharmacokinetic-pharmacodynamic (PK-PD) modelling (2).
Method: Data from humans and rats were collected from published literature on drugs known to cause sedation in the clinic. PK data were based on Cmax, and expressed as free plasma concentration in nM. PD data were standardised to a 0-3 score, where 0 presents no sedation, 1 represents mild (impaired performance but alert), 2 represents moderate (sedated but responsive) and 3 represents substantial sedation (unresponsive). Where PK and PD data were not measured in the same study, PK was extrapolated from published data via the same route of administration. To quantify the PK-PD relationship, the PD scores were transformed to a binary scale and set across all drugs to be simultaneously fitted using a logistic regression model to enable the plasma concentration, which produced a 50% probability of moderate to substantial sedation (scores >1, “SED50”) to be estimated for each drug.
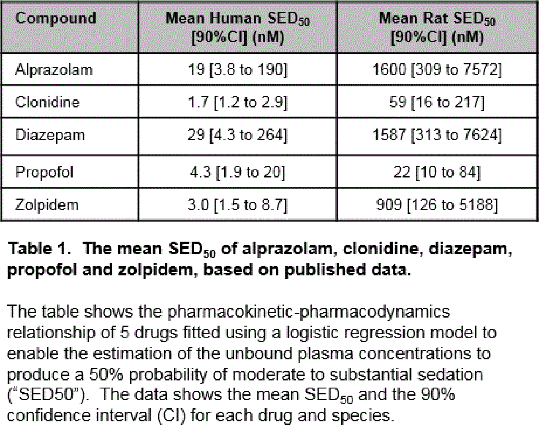
Results: From a list of drugs known to cause sedation in humans, 15 were identified with suitable data and all caused sedation in rats. Only 5 drugs (alprazolam, clonidine, diazepam, propofol and zolpidem) had complete published datasets for both human and rat PK-PD parameters. All these drugs, except for propofol, showed apparent rightward shifts of the concentration-response curves in rats compared to humans. Using the logistic regression model, the mean SED50 for all 5 drugs were determined (Table 1). The rightward shift factor of the mean SED50 from human to rats for alprazolam, clonidine, diazepam, propofol and zolpidem were 84x, 35x, 55x, 5x and 303x, respectively.
Conclusions: Rat models can predict pharmacologically-induced sedation in humans. However, based on drug exposures, the effects of sedation in the rat can underestimate the effects in humans. Therefore, rat models will require testing at higher exposures of sedative drugs in order to predict effects in humans with confidence.
References:
(1) Miller DD (2004) Primary care companion to the Journal of clinical psychiatry 6: 3.
(2) Li J et al. (2011) Predictive Toxicology in Drug Safety. Publisher: Cambridge Univ Pr UK.