Print version
Search Pub Med
| 081P London, UK Pharmacology 2017 |
Predicting pharmacokinetics following topical application using non-animal methods
Introduction: Physiologically Based Pharmacokinetic (PBPK) models are routinely used to predict human kinetic profiles of compounds. The ADME information required to build these models can be generated using differing approaches including in silico, in vitro, and extrapolation from animal models. We present an evaluation of the accuracy of current non-animal methods for predicting human pharmacokinetics of topically applied compounds. Hepatic metabolism data was generated in four different in vitro model systems using primary human hepatocytes (PHH) to investigate the most appropriate system to study low clearance compounds. Dermal absorption rate was estimated by both in silico and in vitro approaches.
Methods: Predictions of plasma profiles following topical application were generated using Gastroplus 9.0 PBPK software for six compounds (diclofenac, salicylic acid, coumarin, nicotine, caffeine and DEET). These predictions corresponded to published clinical studies. Two methods for predicting dermal absorption rate were used, the in silico Gastroplus dermal module and ex-vivo human skin penetration studies. Hepatic clearance was determined using PHH suspension cultures. In vitro hepatic clearance rates using PHH (suspension, collagen coated monoculture, 3D co-cultured microtissues and co-cultured micropatterned hepatocytes) incubated for 4 -168 hours were compared to known in vivo rates of clearance for seven compounds (warfarin, tolbutamide, imipramine, dextromethorphan, salicylic acid, nicotine and diclofenac). The In Sphero human liver spheroid was used as the 3D microtissue system (1). The micropatterned hepatocyte assay was performed by Solvo Biotechnology using the HepatoPac assay (2). Substrate depletion and metabolite formation was measured by LC-MS/MS.
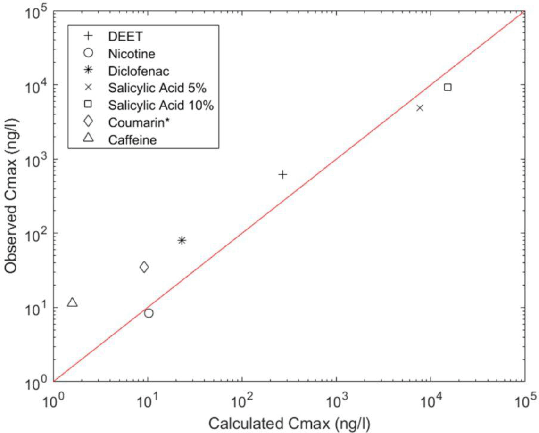
Results: The calculated and clinically observed Cmax values are shown above (R2 = 0.81). The comparison between calculated and observed AUC∞ are of similar accuracy (R2 = 0.84). The HepatoPac assay demonstrated significant substrate depletion not seen in the other systems for salicylic acid, nicotine, warfarin and tolbutamide. Further, both phase I and II metabolites and increased number of metabolites were observed in the co-cultured HepatoPac assay compared to the microtissue assay.
Conclusion: The combination of in vitro data generation and in silico PBPK modelling showed good accuracy for predicting human plasma maximum concentration and AUC values within an order of magnitude for all six compounds. The micropatterned hepatocyte showed promise for measuring the clearance of chemicals that are metabolised too slowly to be detectable in a suspension assay.
References:
(1) Messner S et al. (2013). Archives of Toxicology 87: 209-213.
(2) Ramsden D et al. (2014). Drug Metabolism and Disposition 42: 394-406.

