Print version
Search Pub Med
| 094P London, UK Pharmacology 2017 |
Structure activity relationships of the TASK3 activator Terbinafine and analogues at K2P channels
Introduction: Two-pore domain potassium channels (K2P), or ‘leak’ channels, primarily act to help maintain resting membrane potential (1). Despite a number of important roles, there is currently a lack of selective tools to probe K2P pharmacology. We recently identified Terbinafine as a selective activator of TASK3 (2) and have subsequently assayed Terbinafine and five closely related analogues against TASK3, TWIK1, TRESK, TREK2, TASK2 and THIK1 to gain further insight into K2P channel modulation.
Method: K2P function was measured using thallium flux in transiently-transduced U-2 OS cells, as previously described (2). Tebinafine-hydrocholoride, Naftifine-hydrocholride and Butenafine-hydrochloride (Sigma Aldrich, UK) and Cis-Terbinafine, N-desmethyl-Terbinafine and N-[(2E)-6,6-Dimethyl-2-hepten-4-yn-1-yl]-N,4-dimethyl-1-Naphthalenemethanamine-hydrochloride (Terbinafine Impurity D) (Toronto Research Chemicals, Canada) were screened as 10-pt concentration response curves using the FLIPR Potassium Assay Kit (Molecular Devices).
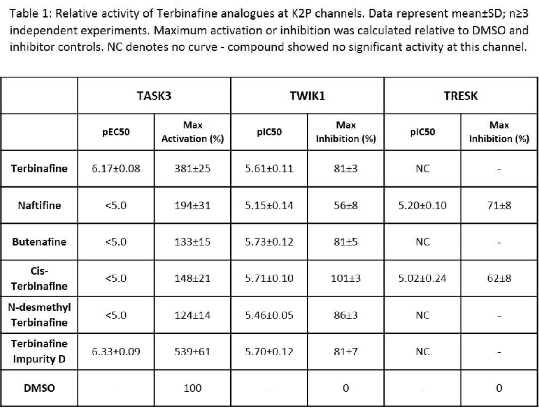
Results: Terbinafine showed robust activation of TASK3 but only one of the analogues tested, Terbinafine Impurity D, activated this channel (Table 1). In contrast, Terbinafine and each of the analogues demonstrated near equipotent inhibition of TWIK1 (Table 1). Two of the analogues, Naftifine and Cis-Terbinafine, showed inhibition of TRESK. Terbinafine and the remaining analogues had limited, if any, activity against TRESK (Table 1). Similarly, limited or no effects (pEC50/pIC50 < 5.0) were observed at TREK2, TASK2 and THIK1.
Conclusions: We have shown that Terbinafine is a highly selective activator of TASK3 in vitro and the complexity in K2P modulation. This data suggests even closely related Terbinafine analogues have divergent pharmacology in the K2P channels studied. We have developed a suite of novel thallium flux assays that are capable of identifying both activators and inhibitors at each of the K2P channels and it is hoped that the tool compounds generated will allow a more complete interrogation of this target class.
References:
(1) Enyedi P and Czirjak G (2010). Physiological Reviews 90: 559-605.
(2) Wright PD et al. (2017). Biochemical and Biophysical Research Communications 493: 444-450.