| 100P London, UK Pharmacology 2017 |
Real time analysis of β-arrestin2 recruitment at the gastrin releasing peptide BB2 receptor using Nanoluciferase complementation
Introduction: Gastrin releasing peptide (GRP) and its G protein coupled receptor BB2 are implicated in pain perception, gastrointestinal regulation and chemoattraction, as well as tumour proliferation (1). While this receptor is reported to have significantly higher affinity (approximately 100 fold) for GRP over the related endogenous peptide neuromedin B (NMB), both GRP and NMB may stimulate BB2 signalling effectively (1). Here we use a new complementation assay (NanoBiT, Promega; 2), based on the modified shrimp luciferase NanoLuc, to compare GRP and NMB stimulated recruitment of the effector β-arrestin2 to the BB2 receptor.
Method: Stably transfected HEK293T cells co-expressed SNAP-tagged BB2 receptors fused at the C terminus to the 156 amino acid NanoLuc fragment (LgBiT), and β-arrestin2 modified at the N terminus with the SmBiT complementary peptide (11 amino acids) and 5 GlySer linker (HEK BB2/A2]. Cell culture and fluo4 calcium mobilisation assays were as described (3). For NanoBiT β-arrestin2 assays, cells in 96 well white walled plates were loaded with furimazine substrate (2) in HBS / 0.1 %BSA 5min prior to agonist for 30min at 37°C. Luminescence was recorded using a BMG Pherastar2 platereader. Calcium and arrestin recruitment concentration response relationships were fitted to individual triplicate experiments using GraphPad Prism v7, and pEC50/Rmax values are mean±s.e.m. from 4–6 experiments.
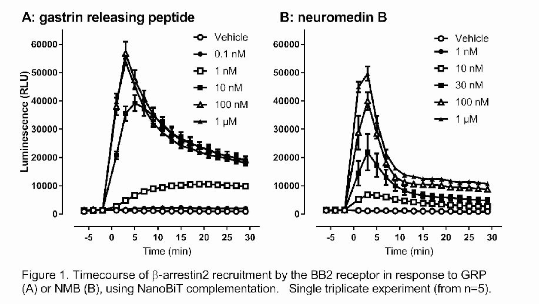
Results: GRP and NMB stimulated peak calcium mobilisation with respective pEC50s of 9.12±0.14 and 8.16±0.19, and equivalent Rmax values (GRP: 2.20±0.11 fold over basal, NMB: 2.19±0.09). Figure 1 demonstrates that each agonist also stimulated BB2 association with β-arrestin2, with similar maximal responses at peak; however recruitment was more transient following NMB addition. Kinetic differences were underpinned by concentration response curves at different time points. At 3min following stimulation, pEC50 values were 7.95±0.10 for GRP and 7.22±0.23 for NMB, with Rmax to NMB being 98.8±10.3% of 100nM GRP. At 29min, the relative potency of GRP increased (pEC509.03±0.19, P<0.05 cf 3min, Student’s t test), while the NMB Rmax declined (57.5±2.9% of 100nM GRP response; P<0.05 cf 3min).
Conclusions: NanoBiT based complementation is a rapid reversible measure of β-arrestin2 association with the BB2 receptor, showing that GRP and NMB activate this pathway with differing response kinetics. Supported by the Nottingham-Brazil CAPES Drug Discovery program and a BPS vacation studentship (YF)
References:
(1) Jensen RT et al. (2008). Pharmacol Rev 60:1-42.
(2) Dixon AS et al. (2016). ACS Chem Biol 11:400-408.
(3) Watson SJ et al. (2012). Mol Pharmacol 81:631-642.