| 126P London, UK Pharmacology 2017 |
In silico simulation of the impact of genetic polymorphisms on long-acting injectable medicines
Background: In recent years, long-acting injectable medicines have been successfully deployed for contraception and schizophrenia, providing therapeutic exposure for a period of months. A long-acting injectable rilpivirine (RPV) formulation has recently been developed for HIV therapy and prevention. Single nucleotide polymorphisms (SNPs) in drug metabolising enzymes and transporter genes are known to influence the oral pharmacokinetics of drugs, but in many cases the effects of such variants are not so profound as to influence posology. The aim of this study was to use physiologically-based pharmacokinetic (PBPK) modelling to predict the influence of theoretical variants on long-acting injectable agents, to assess whether a more profound effect is observed for variants causing relatively small changes in systemic clearance. RPV was used as a model long-acting agent.
Method: The PBPK model was designed in Simbiology v. 4.3.1 (MATLAB 2013b) [1] and 500 healthy adult individuals were used for simulations. The model was qualified against available oral and intramuscular(IM) clinical data (LATTE-2 study) [2]. Different percentage decreases (5-30%) in systemic clearance were simulated in the qualified PBPK model and used to predict the impact upon RPV pharmacokinetics. The models were also used to assess whether different dosing intervals for 600 mg IM doses based upon these variants, using an efficacy cut-off (minimum concentrations to achieve viral suppression) (Ctrough) of 92 ng/ml at steady-state.
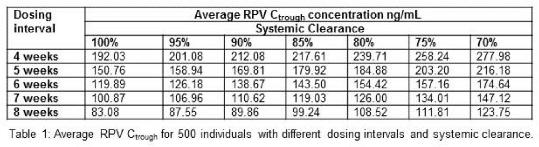
Results: The developed PBPK model was successfully qualified against available oral and IM clinical data, with simulated values ± 50 % from the mean reported values. Simulations indicated that RPV plasma Ctrough would be above the cut-off value for all theoretical variants for 7 weeks, and for 8 weeks for variants causing above a 15% change in systemic clearance (table 1).
Conclusion: These data indicate that even genetic variants causing a minor influence on systemic clearance may have a profound impact on the dosing interval needed for long-acting medicines. Further work is warranted to assess the utility of pharmacogenetic-guided dosing for long-acting medicines used in schizophrenia, contraception and HIV.
References:
1. Rajoli, R.K., et al., Physiologically Based Pharmacokinetic Modelling to Inform Development of Intramuscular Long-Acting Nanoformulations for HIV. Clin Pharmacokinetics, 2015. 54(6): p. 639-50.
2. Margolis, D.A., et al., Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet, 2017. 390(10101): p. 1499-1510.