Print version
Search Pub Med
| 148P London, UK Pharmacology 2017 |
Probe dependent effects on the affinity of the adenosine A3 receptor antagonists
Introduction: G protein-coupled receptors (GPCRs) play a crucial role in mediating cellular responses to external stimuli and are a key target in medical research. Ligand binding to GPCRs can be monitored using a range of techniques, including energy transfer techniques. Recently, bioluminescence resonance energy transfer (BRET) has been used to monitor ligand binding at the human adenosine A3 receptor (A3R) (1). The A3 receptor can form homodimers and orthosteric ligands have been shown to alter the dissociation rate of an orthosteric fluorescent agonist allosterically via the dimer interface (2). To investigate if this dimeric interaction alters the measured affinity of ligands, we have studied the affinity of eight A3 receptor antagonists using three fluorescent antagonist probes.
Methods: HEK293 cells expressing human A3R tagged at the N-terminus with NanoLuc were used in saturation and competition NanoBRET assays as previously described (1). All data are mean ± SEM representative of n experiments performed in triplicate. Statistically significance was determined by one-way analysis of variance (ANOVA, P<0.05 considered significant).
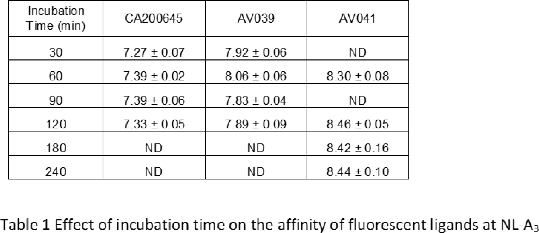
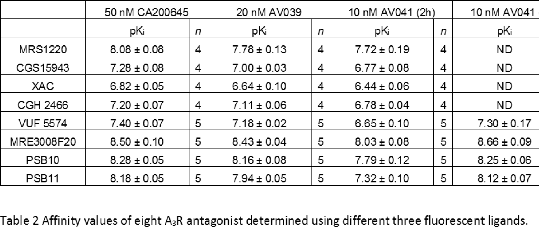
Results: Firstly, to confirm that equilibrium had been reached, saturation binding experiments were performed on the Nluc-A3 cells with BODIPY 630/650 labelled antagonists CA200645 (1), AV039 (3) and XAC-ser-tyr-BY630 (4) at different incubation times (Table 1). There was no significant change in the affinity of these ligands with time. Then, the affinities of eight known A3R antagonists were determined in competition binding assays with a 2 h incubation time using either CA200645, AV039 or XAC-ser-tyr-BY630 as the probe (Table 2). All ligands, apart from MRS1220, displayed significantly higher affinity when using AV039 or CA200645 as the probe compared to XAC-ser-tyr-BY630. To further investigate this for four antagonists, the incubation time was lengthened to 4 hours and initial differences were no longer observed (Table 2).
Conclusions: These data are in agreement with previous NanoBRET data at the human β1-adrenoceptor (5), and further emphasize the importance of ensuring both the fluorescent ligand and competing ligands are at equilibrium before interpretations of probe dependence can be made.
References:
1. Stoddart LA et al. (2015). Nature Methods 3: 661-663
2. May LT et al. (2011). FASB J. 25: 3465-3476
3. Vernall AJ et al. (2012). J Med Chem. 55: 1771-1782
4. Vernall AJ et al. (2013). Org Biomol Chem. 11: 5673-5682 5. Soave M et al. (2016). Pharmacol Res Perspect. 4: e00250