Print version
Search Pub Med
| 153P London, UK Pharmacology 2017 |
The utility of SMALPs for investigating the structure/function of bovine rhodopsin
Introduction: It is inherently difficult to extract integral-membrane proteins for purification and functional characterisation. Styrene maleic anhydride co-polymer (SMA) does this without detergents, preserving annular lipids and has been used to extract G protein-coupled receptors (GPCRs), generating SMA-lipid particles, SMALPs (1, 2, 3). Here we compare SMA with the previously characterised n-dodecyl-β-D-maltopyranoside (DDM) for the extraction of rhodopsin (containing a sensitive chromophore that enables careful observation of its extraction and purification) from rod outer segment (ROS) membranes.
Methods: ROS membranes (4) were incubated in phosphate buffered saline containing 1% DDM or 2.5% SMA in the dark for 1 h, room temperature. Insoluble material was pelleted by centrifugation for 30 min, 20000 x g. Supernatants were analysed by UV-vis absorbance spectroscopy to determine the extent of extraction of rhodopsin. Extracts were buffer exchanged using PD-10 columns into 50 mM Tris, pH 8.0 buffer and incubated with rho1D4-Sepharose beads for 1 h for purification as described previously (5). These methods were applied to HEK 293S GnTI- cells expressing a thermostable rhodopsin mutant. SDS-PAGE (Fig 1) confirmed the purity of the rhodopsin.
Results: SMA is as efficient at extracting rhodopsin as DDM from ROS as indicated by comparable rhodopsin pigment yields (A500nm and SDS-PAGE, Fig 1, 2). Rhodopsin was purified to homogeneity in a single step. Successful purification from HEK 293S cells was also observed. Isomerisation of the 11-cis-retinal is evident upon photobleaching with photoconversion to intermediate states under the conditions used (not shown).
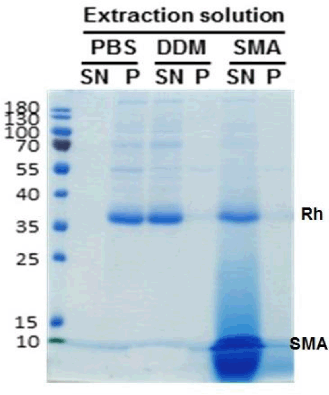
Fig 1. SDS-PAGE gel (representative of 15) of purified rhodopsin (Rh) comparing protein in pellet (P) and supernatant (SN) after extraction with PBS, DDM or SMA (which runs as a diffuse band below rhodopsin).
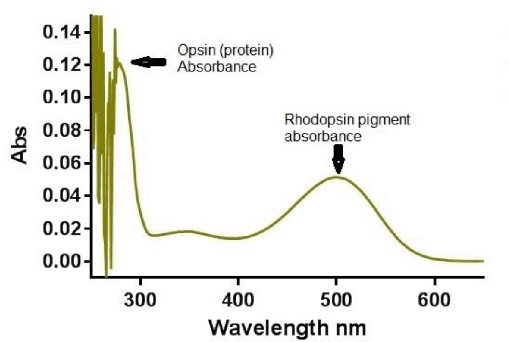
Fig 2. Spectrum of rhodopsin
Conclusions: The development of methods for SMA extraction/purification of rhodopsin from native membranes and cultured cells is demonstrated here. This allows application of these methods to the plethora of rhodopsin mutant constructs to study its activation and other GPCRs in native states.
References:
1, Jamshad M et al., (2015) Bioscience Reports 35: e00188;
2, Wheatley M et al., (2016) Biochemical Society Transactions 44: 619-23;
3, Knowles TJ et al., (2009) Journal of the American Chemical Society 131: 7484-5;
4, Papermaster DS (1982) Methods in Enzymology 81: 48-52; 5, Opefi CA et al., (2013) Journal of Biological Chemistry 288: 33912-33926
We acknowledge financial support from MRC-CiC award (MC_PC_15032) to MW plus BBSRC joint grants BB/I020349/1 and BB/I019960/1 to MW and DRP respectively.

