Print version
Search Pub Med
- A. Franke1
- 2
- J. L. Bryant3
- G. B. Ryan3
- M. Hartley3
- R. J. Spruce3
- H. Mehanna3
- N. Batis3. 1Universitaet zu Luebeck
- Luebeck
- Germany
- 2InHANSE
- Institute of Cancer and Genetic Sciences
- University of Birmingham
- Birmingham
- United Kingdom
- 3InHANSE
- Institute of Cancer and Genomic Sciences
- University of Birmingham
- Birmingham
- United Kingdom
| 161P London, UK Pharmacology 2017 |
The repurposed drug IHN109 identified in the AcceleraTED platform is a promising candidate for treatment of Head and Neck cancer
Introduction: Worldwide, more than 300 000 people die from head and neck cancer (HNC) each year making it the 6th most common type of cancer (1). The considerable side effects of current treatment and poor prognosis highlight the urgent need for novel treatments. The AcceleraTED platform has proven to be a useful tool for identifying repurposed and new drugs in treating HNC. It consists of several stages; Stage 1: high throughput screening assays on a representative HNC cell line panel; Stage 2: validation by additional in vitro assays on both cell lines and primary cultures; Stage 3: validation of in vitro findings using xenograft animal models; Stage 4: initiating and running early to late phase clinical trials. The antimalarial drug IHN109 has been identified as a positive hit in stage 1 and was further proceeded through the platform. The overall aims of this study were to identify a lead drug in the AcceleraTED platform and to show its in vitro and in vivo anti-cancer activity.
Methods: Quantitative measurements of cell proliferation were performed using the AlamarBlue cell viability assay. Cells were seeded at appropriate optimised cell densities in 96 well-plates 24hrs prior to drug treatment. Appropriate drug treatments were added to triplicate wells and incubated for 68hrs at 37oC. 4hrs prior to measurement, 20μl AlamarBlue reagent was added per well and cells were further incubated at 37oC. Measurements were performed using 550/590nm fluorescence counter. All RFU values were normalised by subtracting values from paired media-only plates. The concentrations used for IHN109 range from 1.3x10-7M-8x10-6M.
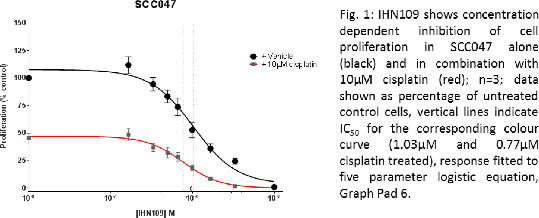
Results: We demonstrate that IHN109 is able to reduce cell viability in 3 different cell lines (HPV-negative CAL27, SCC040; HPV-positive SCC047) in a concentration dependent manner alone and in combination with cisplatin (Fig.1; n=3). IHN109 enhances cisplatin’s inhibitory effect on cell viability in established cell lines and patient derived primary cells (Fig.2; n=3-4) in a concentration dependent manner; whilst maintaining cisplatin’s concentration dependent effect. Moreover, IHN109 exhibits anti-colony forming potential comparable to standard of care treatment cisplatin and irradiation (data not shown).
Summary: IHN109 is a promising drug for the treatment of HNC. We established its anti-viability potential in both HNC cell lines and primary cells, as well as its anti-colony forming activity in a concentration dependent manner. The potential mode of action and in vivo tolerability are currently being investigated.
References:
(1) Fung C and Grandis JR (2010). Expert Opin Emerg Drugs 15(3): 355-373.
.gif)

