Print version
Search Pub Med
| 206P London, UK Pharmacology 2017 |
Harnessing herbs to potentiate P2X7: elucidating the binding site of ginsenoside CK on P2X7
Introduction: Recent work highlighted that the metabolite compound K (CK), from the traditional Chinese herb Panax ginseng, functions as a positive allosteric modulator of P2X7 (1). This evoked sustained intracellular calcium release and enhanced cell death in macrophages; a mechanism which could be exploited for removal of intracellular pathogens. Therefore, we aimed to delineate the binding site for CK within P2X7 to understand the mechanism of potentiation and ultimately exploit its antimicrobial potential.
Methods: A molecular modelling approach was utilized to identify potential binding regions within human P2X7 (hP2X7) using a homology model based on zebrafish P2X4 (2). Amino acid residues predicted to interact with CK were mutated using site-directed mutagenesis and stably-transfected HEK293 cell lines expressing the mutant receptors were generated (HEK-hP2X7). YOPRO-1 assays and whole-cell patch clamp electrophysiology were conducted, as described previously (1). Data were analysed for statistical significance using either unpaired t-tests or one-way ANOVA with post-tests as appropriate.
Results: Molecular docking predicted CK to associate with residues found within the central vestibule of P2X7. Consequently, HEK293 cells were transfected with receptors containing mutations in these residues (labelled Mid 1 to Mid 14). The dose response to ATP was left-shifted in Mid 5, Mid 8, and Mid 14, indicating increased sensitivity to ATP (EC50 of <50 μM for all the mutants, compared to 266 μM for WT). Moreover, these responses were inhibited by the selective P2X7 antagonists AZ10606120 and AZ11645373 (both 10 μM). Mid 5 and Mid 8 also lost much of the potentiation elicited by CK (10 μM) whilst maintaining sensitivity to ATP stimulation. This was demonstrated by decreased uptake of the cell impermeable dye YOPRO-1 (fold change in response to a combination of ATP and CK versus ATP alone, of 0.24 and 0.55 for Mid 5 and Mid 8, respectively, compared to 3.95 for WT; p<0.05; n=3). These observations were further supported by whole-cell patch clamp recordings, which additionally highlighted a significant decrease in the magnitude of potentiation by CK for the Mid 1 and Mid 3 mutations when compared to WT hP2X7 (Fig. 1; *p<0.05; n=5-9 cells).
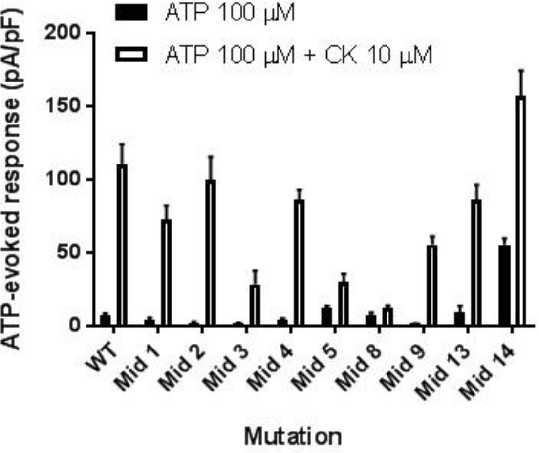
Conclusions: To conclude, we have identified promising amino acid residues that contribute to the positive allosteric effects of CK on ATP-induced responses by hP2X7, but further mutagenesis is required to fully elucidate the complete binding site.
References
1) Helliwell, R.M., et al. Br J Pharmacol, 2015, 172(13), 3326-40.
2) Kawate, T. et al. Nature, 2009, 460 (7255), 592-598.

