| 005P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Triazoloquinazolines: dual-target, a2ar - pde 10a, anti-proliferative compounds
Introduction: Activation of the Adenosine A2A receptor (A2AR), and inhibition of phosphodiesterases, has been show to be anti-proliferative [1]. Using in silico docking, we identified a series of known PDE 10A inhibitors, triazoloquinazolines [2], which displayed promising docking scores to the orthosteric agonist-binding site of the A2AR. Here we present data pertaining to the characterisation of these compounds with respect to activation of the A2AR, identifying their selectivity profiles amongst adenosine receptors, as well as identifying their dual-target action to also be anti-proliferative.
Methods: Test compounds were initially screened in yeast expressing either the A1, A2A or A2B receptors [3]. cAMP accumulation/inhibition was measured using a LANCE cAMP kit (Perkin Elmer), for CHO-K1-A2AR and CHO-K1-A3R cells, as well as C6 and HEK 293S cells. All cell types were stimulated with agonist (10 mM - 10 pM) for 30 minutes prior to determining intracellular cAMP concentrations. For proliferation assays, cells were seeded onto 96 well plates at a density of 2000 cells per well, and treated with agonists after 24 hours, for a period of 72 hours. Cell number was then quantified using CCK-8 (Sigma Aldrich).
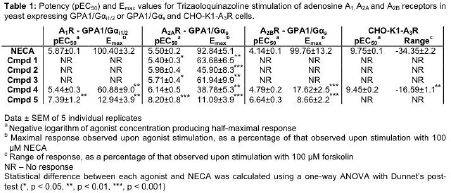
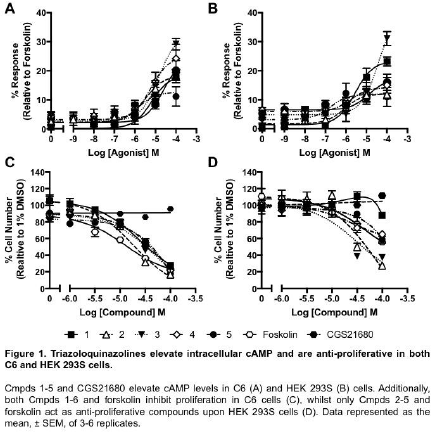
Results: Screening of test compounds (Cmpds) 1-5: in yeast (β-galactosidase), CHO-K1-A2AR and CHO-K1-A3R cells (cAMP accumulation/inhibition), identified the ability of all compounds to act as agonists against the A2AR, with Cmpds 1-3 being selective for the A2AR (Table 1). Further testing in cells endogenously expressing the A2AR and PDE 10A, C6 and HEK 293S cells, identified their abilities to elevate intracellular cAMP levels (Figure 1A-B), as well as inhibit proliferation (Figure 1C-D). This anti-proliferative action is specific to the triazoloquinazoline compounds, as CGS21680 (a selective A2AR agonist) had no effect upon proliferation in either cell type (Figure 1C-D). However, cAMP appears to be the key mediator of these compounds’ actions as forskolin was also found to be anti-proliferative (Figure 1C-D).
Conclusions: We have identified a series of dual action compounds, of the triazoloquinazoline chemical series, that are both A2AR agonists and PDE 10A inhibitors. Testing these compounds in cells endogenously expressing both of these targets identified that our compounds appear to have an anti-proliferative effect, which is cAMP dependent; this been particularly apparent in a rat model of glioma (C6 cells).
References:
[1] Rickles et al (2010). Blood. 116:593-602.
[2] Kehler et al (2011). Bioorg. Med. Chem. Lett. 21:3738-42;
[3] Knight et al (2016). J. Med. Chem. 59:947-64.