| 016P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Receptor Component Protein (RCP) as an regulator of agonist bias at the CGRP receptor
Introduction: RCP is a 148 amino acid, 17 kDa peripheral membrane protein expressed in numerous immortalized cell lines (1). It is found in vivo in the brain, spinal cord, the uterus as well as in vasculature. It is required for efficient coupling of the calcitonin gene-related peptide (CGRP) receptor to production of cAMP via Gαs, the stimulatory G protein. Functional CGRP receptors consist of a 7-transmembrane domain receptor, the calcitonin receptor-like receptor (CLR). This requires an accessory protein, receptor activity modifying protein 1 (RAMP1) for ligand binding and receptor expression (2). RCP is a third component of the receptor. It appears to physically associate with the receptor, interacting with its second intracellular loop (ICL2) (3). In this study, we have examined the role of RCP on a variety of signalling pathways that are activated by the CGRP receptor.
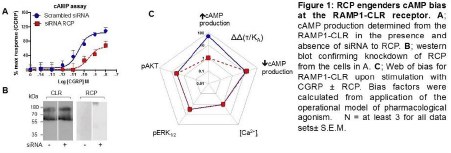
Methods: HEK293T cells were transfected with 10 nM siRNA (Sigma) to knock down endogenous RCP expression (Fig 1), and were transfected 48 h later with CLR and RAMP1 as described previously (4). Cells were stimulated with human alpha CGRP (10-14 to 10-6M) and cAMP (LANCE kits, Perkin Elmer), pERK and pAtk-1 (CISBIO kits) were determined. Intracellular calcium was measured via FURA-4AM. Concentration-response curves were analysed via PRISM 7 according to the operational model to obtain log(tau) and log(KA), from which bias plots were constructed (4). All experiments were repeated in triplicate.
Results: CGRP stimulated cAMP production (pEC50 10.5±0.12), pAtk (pEC50 8.5±0.24) and pERK phosphorylation (pEC50 9.44±0.27) and increased intracellular calcium (pEC50 6.8±0.20). There were no differences in the responses in the presence of siRNA against RCP to pAtk, pERK or calcium, but for cAMP, the Emax was reduced by 57% and the pEC50 was reduced to 9.6±0.18 (Fig 1). Cell surface expression of CLR was unaffected as determined by ELISA (135±35% of control). Homology modelling suggested that RCP interacts with the αB-αC loop of Gαs that shows little homology to other G proteins.
Conclusions: RCP appears to be an allosteric modulator of CLR that specifically influences agonist bias by selectively enhancing coupling to Gs. This work was supported by BBSRC grants BB/M000176/1 and BB/M00015X/1.
References:
1) Dickerson, I.M., (2013) Curr Protein Pept Sci 14, 407-15
2) McLatchie, L.M. et al., (1998) Nature 393, 333-339
3) Egea S.C. and Dickerson I.M. (2012) Endocrinology 153,1850-60
4) Weston C. et al., J. Biol Chem. (2016) 291, 21925-21944
Figure 1 Actions of RCP