Print version
Search Pub Med
| 018P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Photoaffinity crosslinking defines the base of the CGRP binding site to its receptor
Introduction: Calcitonin gene related peptide (CGRP) is a very abundant neuropeptide, especially in sensory nerve fibres. We have employed an unnatural amino acid approach to understand how the CGRP binds to its receptor, a complex between calcitonin receptor-like receptor (CLR), a G protein coupled receptor and receptor activity modifying protein 1 (RAMP1) (1). Previously we substituted each of the residues in extracellular loop 2 (ECL2) with the amber stop codon and genetically incorporated azido-phenylalanine (AzF) into the receptor structure. Subsequent UV exposure of the bound complex demonstrated that residues 284 and 291 crosslinked to CGRP (Simms et al., presented at Pharmacology 2017). In this study, we have attempted to further delineate the binding pocket by substituting M223 in helix 3 and F349 in helix 7 with AzF.
Methods: Unnatural amino acid mutagenesis was performed in HEK293T cells as described previously, using amber mutant human CLR, RAMP1, suppressor tRNA, and azF amino-acyl tRNA synthetase (2). Membranes from the cells were incubated with 100nM human alpha CGRP (with position 15 replaced by fluorescein) for 60min at room temperature. Samples (1ml) were put into quartz cuvettes and either exposed to 300nm light for 1hr or wrapped in aluminium foil and also placed in the light source. Samples were solubilised with 1% DDM for 4 hours, centrifuged and 0.15ml aliquots measured for fluorescence.
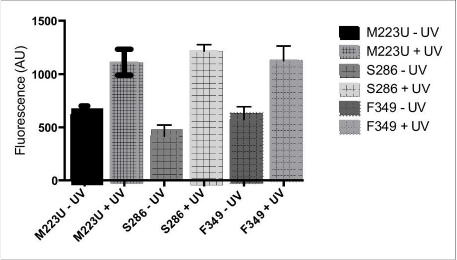
Results: We were able to show that cross-linking occurred with both AzF mutants. The amount of label incorporated was comparable to that seen when S286 is substituted with AzF, around 500 fluorescence absorbance units. Whilst for each mutant this was significantly different from the non-crosslinked controls (Student’s t-test, p<0.05, n=3), when I284 and L291 were substituted with AzF, the labelled was 3.9 and 5.8 fold greater than that seen with substitution of S286.
Conclusions: It seems that M230 and F349 form the base of the ligand binding pocket for the CGRP receptor but they are minor contacts compared to I284 and L291 in ECL2. DRP and JS were supported by BBSRC grant BB/M007529/1
References:
1) McLatchie. L.M. et al., (1998), Nature, 393, 333-339
2) Koole, C., et al., (2017), J. Biol. Chem., 292: 7131-7144
Figure 1 Cross-linking of 15-Fluo CGRP to mutant receptors. Data compares membranes with or without exposure to uv light.